Admission to the intensive care unit (ICU) is a standard of care after transcatheter aortic valve implantation (TAVI); however, the improvement of the procedure and the need to minimize the unnecessary use of medical resources call into question this strategy. We evaluated prospectively 177 consecutive patients who underwent TAVI. Low-risk patients, admitted to conventional cardiology units, had stable clinical state, transfemoral access, no right bundle branch block, permanent pacing with a self-expandable valve, and no complication occurring during the procedure. High-risk patients included all the others transferred to ICU. In-hospital events were the primary end point (Valve Academic Research Consortium 2 criteria). The mean age of patients was 83.5 ± 6.5 years, and the mean logistic EuroSCORE was 14.6 ± 9.7%. The balloon-expandable SAPIEN 3 valve was mainly used (n = 148; 83.6%), mostly with transfemoral access (n = 167; 94.4%). Among the 61 patients (34.5%) included in the low-risk group, only 1 (1.6%) had a minor complication (negative predictive value 98.4%, 95% confidence interval [CI] 0.91 to 0.99). Conversely, 31 patients (26.7%) from the high-risk group had clinical events (positive predictive value 26.7%, 95% CI 0.19 to 0.35), mainly conductive disorders requiring pacemaker (n = 26; 14.7%). In multivariate analysis, right bundle branch block (odds ratio [OR] 14.1, 95% CI 3.5 to 56.3), use of the self-expandable valve without a pacemaker (OR 5.5, 95% CI 2 to 16.3), vitamin K antagonist treatment (OR 3.8, 95% CI 1.1 to 12.6), and female gender (OR 2.6, 95% CI 1.003 to 6.9) were preprocedural predictive factors of adverse events. In conclusion, our results suggested that TAVI can be performed safely without ICU admission in selected patients. This strategy may optimize efficiency and cost-effectiveness of procedures.
Transcatheter aortic valve implantation (TAVI) is now the standard of care for inoperable patients with severe symptomatic aortic stenosis and an accepted alternative to surgery for high-risk patients. TAVI is less invasive and associated with fewer complications than the traditional approach for aortic valve replacement through median sternotomy and cardiopulmonary bypass. However, TAVI is an interventional technique with specific postoperative complications related to the procedure itself but also to multiple co-morbidities of the patients. These complications were standardized and defined by the Valve Academic Research Consortium (VARC-2), updated in 2013. With the improvement in operator experience and valve technology, combined with better screening and trend to include lower risk patients, contemporary studies have shown a dramatic decrease of in-hospital complications after TAVI. Recent reports showed the possibility to decrease hospital length of stay in selected patients with subsequent decrease of procedural costs. Although there are currently no specific guidelines concerning immediate medical care after TAVI, admission to intensive care unit (ICU) with monitoring has been regarded as an essential step for the patients after the procedure. However, both the improvement of the results of the procedure and the risk of overload of the ICU associated with the development of TAVI call into question this systematic strategy. The objective of this prospective cohort study was to evaluate feasibility and safety of TAVI performed without subsequent ICU admission in patients carefully selected using simple clinical, electrocardiographic (ECG), and echocardiographic selection criteria.
Methods
Between December 2014 and July 2015, all consecutive patients who underwent TAVI in our center were included in a monocentric prospective study. Exclusion criteria included valve-in-valve procedures and transaortic or transapical approaches. The procedures were performed in the catheterization laboratory using the new-generation balloon-expandable Edwards SAPIEN 3 bovine pericardial device (Edwards Lifesciences, Irvine, California) or the self-expandable CoreValve porcine pericardial device (Medtronic, Inc., Minneapolis, Minnesota). All patients had severe symptomatic aortic stenosis secondary to degenerative disease confirmed by transthoracic echocardiography (TTE) (mean gradient >40 mm Hg and/or valve area <1 cm 2 ) and were not candidates for surgical aortic valve replacement according to guidelines and after internal discussions about the therapeutic options within the multidisciplinary heart team. The choice of the valve was left to the decision of the interventional cardiologist. The self-expandable valve was usually preferred in small aortic annulus considering the good hemodynamic performances of this prosthesis or to avoid balloon inflation in case of high risk of annulus rupture (mechanical compression of a “vulnerable area” by calcifications). Patients were divided into 2 groups (low or high risk) according to the presence or absence of risk factors of severe in-hospital complications requiring admission to ICU. Preprocedural and postprocedural simple clinical, ECG, and TTE selection criteria ( Table 1 ) were recorded prospectively. Of note, the use of self-expandable prosthesis in patients without previous pacemaker and the occurrence of any new conductive disorder during the procedure, including PR interval >200 ms, QRS >120 ms, QRS widening >10 ms, or complete atrioventricular block (AVB) were considered as high-risk criteria. For patients included in the low-risk group, clinical events were collected with monitoring during 2 hours after the procedure in an anesthesia recovery room, and then, when no complications occurred, they were admitted to the conventional cardiology unit (CCU) for standard medical care without monitoring. Patients included in the high-risk group were transferred to the ICU for at least 24 hours. The ICU in our center is a 16-bed unit with a full-time staff composed of 6 certified physicians and 1/3 nurse per patient ratio. The ICU allowed ECG monitoring and hemodynamic support, if necessary, and only noninvasive ventilation assistance. The CCU is a standard cardiology unit with 1/15 nurse per patient ratio (1/30 during the night) and no ECG monitoring. Importantly, the final clinical decision to transfer the patient to ICU was left to the physician (cardiologist or anesthetist), even if it concerned a “low-risk” patient according to our predetermined criteria. During hospital stay, ECG was recorded daily. TTE was performed at the admission to ICU in the high-risk group and before hospital discharge in all patients. Permanent pacing requirements were considered according to European Society of Cardiology (ESC) guidelines 2013. Briefly, in case of high-degree or complete AVB, permanent pacemakers were most of the time implanted between the first and the fifth day. In case of persistent new left (LBBB) or right bundle branch block (RBBB), an electrophysiological study was performed between day 2 and day 5, and permanent pacing was considered when His ventricle interval was >60 ms. A combination of clopidogrel 75 mg and aspirin 75 mg was introduced in all patients after the procedure except in those with an indication of vitamin K antagonists (VKAs) or direct oral anticoagulant therapy who had only aspirin 75 mg. The primary end point of the study included in-hospital events considered as requiring ICU admission, with reference to the VARC-2 criteria ( Table 2 ). Medical decision to secondarily transfer a patient initially admitted in the CCU to the ICU was considered as an event regardless of the reason of the transfer. Our objective was firstly to validate the safety of the strategy of TAVI performed without subsequent admission to ICU (negative predictive value [NPV]). Incidence of major adverse events was also evaluated in the 2 groups (positive predictive value), and predictive factors of in-hospital complications requiring ICU admission were assessed to validate our triage strategy. We also evaluated incidence of minor complications (not requiring ICU admission) and duration of hospitalization in the 2 groups of patients. Adverse events were also compared between the 2 implanted prostheses. Patients’ characteristics are presented using mean and SD for continuous variables and frequencies and proportions for categorical variables. Groups (high vs low risk) were compared using a Student t test or Mann-Whitney Wilcoxon rank test for continuous variables, depending on the normality of each variable’s distribution as attested by a Shapiro-Wilk test and chi-square or Fisher exact test for categorical ones. To determine the relative importance of the preprocedural and periprocedural covariates on the occurrence of major complications (VARC-2 criteria), a multivariate analysis using logistic regression was performed. A stepwise selection of the variables was then used, with an alpha-to-enter and alpha-to-exit, respectively, set at 0.15 and 0.05. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated along with p values. The significance of adding or removing a variable from the logistic model was determined by the maximum likelihood ratio test. The goodness-of-fit of the models was assessed using the Hosmer and Lemeshow chi-square test. Statistical bilateral significance threshold was set at 5%. Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Cary, North Carolina).
| Low-risk group | High-risk group | |
|---|---|---|
| Before procedure | ||
| Preexisting pulmonary disease with oxygen dependence. | – | + |
| Permanent pace-maker for patients with CoreValve | + | – |
| Hemodynamic state | Stable | Unstable |
| Complete right bundle branch block (QRS width >120 ms) | – | + |
| Left ventricular ejection fraction > 40% | + | – |
| Systolic arterial pulmonary pressure < 60 mmHg (according to Euroscore definition) | + | – |
| Transfemoral approach | + | – |
| During and 2 hours after procedure | ||
| Hemodynamic state | Stable | Unstable |
| Thrombotic/embolic complications | – | + |
| Minor or major vascular complication (according to VARC 2 definitions) | – | + |
| New conductive disorder | – | + |
| New atrial or ventricular arrhythmia | – | |
| Good positioning of the prosthesis and Aortic regurgitation ≤2 | + | – |
| Medical decision | – | + |
| Complications | Clinical events considered |
|---|---|
| Death | Death from any cause |
| Hemodynamic instability | Mean arterial pressure <65 mm Hg inquiring volume replacement or vasopressors Acute pulmonary edema |
| Major, life threatening or fatal bleeding | Loss of hemoglobin level of at least 3.0 g/dL with hypovolemic shock Transfusion of 2 or more blood units |
| Major and minor vascular complications | Vascular dissection Vascular rupture Surgical or endovascular or percutaneous intervention/repair |
| Stroke | Transient ischemic attack or stroke of any cause |
| Pacemaker requiring | All new conduction defect requiring permanent or transient pacemaker implantation |
| Acute myocardial infarction < 72 hours | Ischemic symptoms or ECG suggestive of ischemia with elevation of cardiac biomarkers (peak value exceeding 15x as the upper reference limit for troponin) |
| Acute kidney injury (Stage 2 or 3 according to RANKIN classification) | Oligoanuria between 12h and 24h Anuria for > 12h Increase in serum creatinine of more than 200% compared with baseline |
| Pericardial effusion | Requiring medical intervention |
| Secondary Transfer to intensive care unit | Any medical reason (physician decision) |
Results
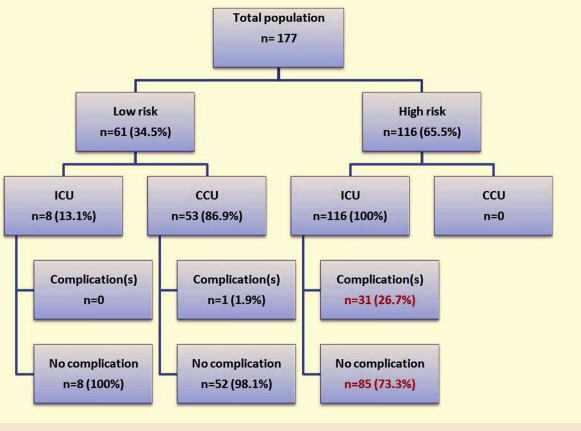
The study enrolled 177 patients, 61 (34.5%) were classified in the low-risk group and 116 (65.5%) in the high-risk group according to the predefined criteria. Except for the risk factors in the criteria defining low- versus high-risk groups in our study, the clinical characteristics between the groups of patients only differed by the logistic EuroSCORE (p = 0.011; Table 3 ). Most TAVIs were performed under general anesthesia with orotracheal intubation (n = 170, 96.1%) and with transfemoral approach (n = 167, 94%). We did not usually use echocardiography for position or deployment of the valve. The SAPIEN 3 prosthesis was mainly used (n = 148, 83.6%). Surgical access was exclusive in our center. Considering their clinical preprocedural characteristics, 76 patients (42.9%) were directly assigned to the high-risk group and transferred to ICU regardless of the course of the procedure ( Figure 1 ). The 3 main reasons were left ventricular ejection fraction <40% (n = 31), CoreValve implantation without previous pacemaker (n = 25), and complete RBBB (n = 14). During and until 2 hours after the procedure, 40 additional patients (22.6%) converted from low- to high-risk group because of occurrence of predefined complications requiring ICU transfer, mainly new conductive disturbance (n = 34, 85%). Among the 116 patients of the high-risk group, 73 (62.9%) developed at least 1 conductive disorder during the procedure (p <0.001; Table 4 ). We observed 8 patients who did not have any complication or high-risk criteria but who were nevertheless transferred to ICU after decision of the cardiologist or the anesthetist owing to frailty ( Figure 1 ). Postprocedural outcome of the population is presented in Table 5 . According to our study end points, 32 patients (18.1%) experienced ≥1 clinical events, mostly represented by conduction disturbances requiring permanent or transient pacing (n = 26; 14.7%). Major vascular complications or bleeding were observed in 2 patients (1.1%). One patient died because of major bleeding. All these events occurred exclusively in patients admitted to ICU and included in the high-risk group. No major complication occurred in the low-risk group. Only 1 patient developed a noncompressive pericardial effusion (<10 mm on TTE) and not defined as a significant event in our predefined criteria. According to medical decision, the patient was transferred to ICU and could go back to CCU 12 hours later. Considering these results, the NPV of our decision-making criteria was 98.4% (95% CI 0.91 to 0.99) with a sensitivity of 96.9% (95% CI 0.84 to 0.99), whereas the positive predictive value was 26.7% (95% CI 0.19 to 0.36) with a specificity of 41.4% (95% CI 0.32 to 0.49). Minor complications, not included in our end point criteria, had favorable evolution and did not require secondary transfer to ICU. Manual compression controlled by Doppler was required for minor vascular events (low-risk group: n = 4; 1.6% and high-risk group: n = 15; 1.3%, p = 0.12). aortic regurgitation occurred, respectively in 43 (24.3%) and 16 (9%) patients of the low- and high-risk group (p = 0.58). Although the risk level did not significantly influence the length of hospital stay in our study ( Table 3 ), patients who developed a complication stayed longer than patients who did not (5.4 ± 3.6 vs 3.7 ± 1.8 days, respectively; p <0.001). Some patients of the high-risk group developed at least 1 mild or transient conductive disorder (mostly LBBB) during monitoring in the ICU that did not require pacing (n = 20; 11.3%). The predictive factors of complications requiring ICU are presented in Table 6 . In multivariate analysis, use of the self-expandable valve without a pacemaker (OR 5.5, 95% CI 2 to 16.3), RBBB (OR 14.1, 95% CI 3.5 to 56.3), VKA treatment (OR 3.8, 95% CI 1.1 to 12.6), and female gender (OR 2.6, 95% CI 1.003 to 6.9) were preprocedural predictive factors of in-hospital adverse events, whereas periprocedural occurrence of LBBB (OR 8.8, 95% CI 2.7 to 28.8) and complete AVB (OR 19.7, 95% CI 5 to 77.3) were highly predictive of complications during ICU stay (p <0.001). As expected, prevalence of permanent pacemaker requirement was higher with the self-expandable CoreValve compared with the balloon-expandable SAPIEN 3 valve (n = 11, 44% vs n = 15, 11.8%, respectively; p <0.0001).
| Variables | Total population (n=177) | Low risk group (34%, n=61) | High risk group (66%, n=116) | P value |
|---|---|---|---|---|
| Age (years) | 83.5 +/- 6.9 | 84.2 +/- 6.3 | 83.1 +/- 7.1 | 0.312 |
| Male gender | 87 (49.2%) | 29 (47.5%) | 58 (50%) | 0.756 |
| Body Mass Index (kg/m 2 ) | 26.3 +/- 6.1 | 26.3 +/- 4.5 | 26.3 +/- 6.9 | 0.362 |
| Euroscore II (%) | 5.3 +/- 4.2 | 4.3 +/- 2.4 | 5.9 +/- 4.8 | 0.028 |
| Logistic Euroscore (%) | 14.6 +/- 9.7 | 12.2 +/- 6.3 | 15.9 +/- 10.9 | 0.011 |
| Hypertension | 114 (64.4%) | 42 (68.9%) | 72 (62.1%) | 0.370 |
| Diabetes mellitus | 49 (27.7%) | 19 (31.2%) | 30 (25.9%) | 0.455 |
| Active smoker | 3 (1.7%) | 1 (1.6%) | 2 (1.7%) | 1.000 |
| Pulmonary disease with O2 dependence | 9 (5.1%) | 0 | 9 (7.8%) | 0.026 |
| Systolic PAP > 60mmHg | 10 (5.7%) | 0 | 10 (8.6%) | 0.018 |
| Coronary artery disease | 87 (49.2%) | 29 (47.5%) | 58 (50%) | 0.755 |
| Peripheral artery disease | 14 (7.9%) | 4 (6.6%) | 10 (8.6%) | 0.629 |
| Atrial fibrillation | 50 (28.3%) | 13 (21.3%) | 37 (31.9%) | 0.137 |
| Cirrhosis | 6 (3.4%) | 2 (3.3%) | 4 (3.5%) | 1.000 |
| NYHA | 0.099 | |||
| I | 16 (9.4%) | 4 (6.8%) | 12 (10.7%) | – |
| II | 46 (26.9%) | 19 (32.2%) | 27 (24.1%) | – |
| III | 87 (50.9%) | 33 (55.9%) | 54 (48.2%) | – |
| IV | 22 (12.9%) | 3 (5.1%) | 19 (17.0%) | – |
| ECG | ||||
| First degree AVB | 24 (13.6%) | 9 (14.8%) | 15 (12.9%) | 0.736 |
| Complete LBBB | 13 (7.3%) | 5 (8.2%) | 8 (6.9%) | 0.767 |
| Complete RBBB | 14 (7.9%) | 0 | 14 (12.1%) | 0.005 |
| Treatment | ||||
| Vitamin K antagonists | 20 (11.3%) | 3 (4.9%) | 17 (14.7%) | 0.052 |
| Dual antiplatelet (aspirin + clopidogrel) | 46 (26.0%) | 16 (26.2%) | 30 (25.9%) | 0.958 |
| Direct oral anticoagulants | 7 (4.0%) | 2 (3.3%) | 5 (4.3%) | 1.000 |
| Dual antiplatelet + Vitamin K antagonists | 16 (9.0%) | 5 (8.2%) | 11 (9.5%) | 0.777 |
| Left ventricular ejection fraction | <0.001 | |||
| < 30% | 2 (6.8%) | 0 | 12 (10.3%) | – |
| 30-40% | 19 (10.7%) | 0 | 19 (16.4%) | – |
| >40% | 146 (82.3%) | 61 (100%) | 85 (73.3%) | – |
| Pacemaker | 25 (14.1%) | 12 (19.7%) | 13 (11.2%) | 0.124 |
| Creatinine clearance (mL/min) | 50.2 +/- 19.6 | 50.0 +/- 18.2 | 50.3 +/- 20.4 | 0.936 |
| Hemoglobin (g/dL) | 12.3 +/- 1.6 | 12.3 +/- 1.7 | 12.3 +/- 1.6 | 0.833 |
| CRP (g/l) | 10.7 +/- 20.9 | 10.8 +/- 22.7 | 10.7 +/- 20.0 | 0.651 |
| Anesthesia | ||||
| General | 170 (96.1%) | 59 (96.7%) | 111 (95.7%) | – |
| Local | 7 (3.9%) | 2 (3.3%) | 5 (4.3%) | – |
| Procedure length (hours) | 1.37 +/- 0.44 | 1.31 +/- 0.34 | 1.41 +/- 0.49 | 0.295 |
| Access | 0.058 | |||
| Right transfemoral | 44 (24.9%) | 17 (27.9%) | 27 (23.3%) | – |
| Left transfemoral | 123 (69.5%) | 44 (72.1%) | 79 (68.1%) | – |
| Subclavian | 0 | 0 | 0 | – |
| Transcarotid | 10 (5.7%) | 0 | 10 (8.6%) | – |
| Prosthesis | 0.010 | |||
| Edwards SAPIEN 3 | 148 (83.6%) | 57 (93.4%) | 91 (78.5%) | – |
| Medtronic CoreValve | 29 (16.4%) | 4 (6.6%) | 25 (21.6%) | – |
| Variables | Total population (n177) | High-risk group (n=116) | P value |
|---|---|---|---|
| Before procedure | |||
| Pulmonary disease with O2 dependence | 9 (5.1%) | 9 (7.8%) | 0.03 |
| CoreValve prosthesis without pacemaker | 25 (14.1%) | 25 (21.6%) | <0.005 |
| Complete right bundle branch block | 14 (7.9%) | 14 (12.1%) | 0.004 |
| Left ventricular ejection fraction < 40% | 31 (17.5%) | 31 (26.7%) | <0.005 |
| Systolic pulmonary arterial pressure > 60mmHg | 10 (5.6%) | 10 (8.6%) | 0.018 |
| During Procedure | |||
| Unstable hemodynamic | 5 (2.8%) | 5 (4.3%) | 0.17 |
| Mean arterial pressure < 65mmHg + vasopressors | 4 (2.3%) | 4 (3.4%) | 0.299 |
| Mean arterial pressure < 65mmHg + volume replacement | 1 (0.6%) | 1 (0.9%) | 0.545 |
| Vascular complications | 6 (3.4%) | 6 (5.2%) | 0.55 |
| Vascular rupture necessity surgery | 2 (1.1%) | 2 (1.7%) | 0.545 |
| Hematoma with manual compression | 4 (2.3%) | 4 (3.4%) | 0.552 |
| Second prosthesis | 1 (0.6%) | 1 (0.9%) | 1 |
| Stroke | 1 (0.6%) | 1 (0.9%) | 1 |
| New conductive disorder | 73 (41.2%) | 73 (62.9%) | <.000001 |
| Complete atrio ventricular block | 26 (14.7%) | 26 (22.4%) | <.001 |
| Complete left bundle branch block | 49 (27.7%) | 49 (42.2%) | <.001 |
| Complete right bundle branch block | 3 (1.7%) | 3 (2.6%) | 0.55 |
| First degree atrio ventricular block | 15 (8.5%) | 15 (12.9%) | 0.003 |
| Pericardial effusion requiring medical intervention | 1 (0.6%) | 1 (0.9%) | 1 |
| Aortic regurgitation > grade 2 or pericardial effusion on echocardiography | 4 (2.3%) | 4 (3.4%) | 0.299 |
| Supraventricular arrhythmia | 1 (0.6%) | 1 (0.9%) | 1 |
| Ventricular arrhythmia | 1 (0.6%) | 1 (0.9%) | 1 |
| Transfert on medical decision | 8 (4.5%) | 8 (6.9%) | – |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


