Histologic proof of granulomatous inflammation is prerequisite for the diagnosis of cardiac sarcoidosis (CS). Because of the limited sensitivity of endomyocardial biopsy (EMB), confirmation of sarcoidosis often has to be acquired from extracardiac biopsies. We set out to review our experience of F-18-fluorodeoxyglucose positron emission tomography (F-18-FDG PET) in guiding extracardiac tissue biopsies in suspected CS. We included in this work 68 consecutive patients with proved CS who had undergone cardiac F-18-FDG PET with (n = 57) or without whole-body imaging as part of initial diagnostic evaluation. Their hospital charts, imaging studies, and diagnostic biopsies were reviewed in retrospect. Whole-body PET images showed extracardiac foci of abnormally high F-18-FDG uptake in 39 of 57 patients, of whom 38 had involvement of mediastinal lymph nodes (MLN). Parallel F-18-FDG uptake was found in other lymph nodes (n = 10), lungs (n = 9), liver (n = 3), spleen (n = 2), and thyroid gland (n = 1). Adding the mediastinal findings at cardiac PET without whole-body imaging, abnormal F-18-FDG uptake in MLN was found in totally 43 of the 68 patients with CS (63%). Histology of systemic sarcoidosis was known at presentation of cardiac symptoms in 8 patients. Of the 60 patients with missing histology, 24 patients underwent mediastinoscopy for sampling of PET-positive MLN, most often (n = 20) after nondiagnostic EMB; microscopy revealed diagnostic noncaseating granulomatous inflammation in 24 of the 24 cases (sensitivity 100%). In the remaining 36 patients, sarcoidosis histology was confirmed by EMB (n = 30), by biopsy of lungs (n = 2) or peripheral lymph nodes (n = 2), or at autopsy (n = 1) or post-transplantation (n = 1). In conclusion, MLN accumulate F-18-FDG at PET in most patients with CS and provide a highly productive source for diagnostic biopsies either primarily or subsequent to nondiagnostic EMB.
Cardiac sarcoidosis (CS) presents to the cardiology services as an isolated heart condition much more commonly than as one manifestation of a known or clinically evident systemic sarcoidosis. Its main forms of presentation are atrioventricular conduction block, ventricular tachyarrhythmias, and heart failure, either alone or in various combinations. Distinguishing CS from more common myocardial conditions, ischemic or nonischemic, is critical for the patient care yet frequently a tough challenge. The only absolute diagnosis of CS comes from finding typical granulomatous myocarditis in a sample of myocardium without other explanation. However, because of the patchy distribution of sarcoid granulomas, endomyocardial biopsy (EMB) more often misses than hits areas diagnostic of CS resulting in a sensitivity no better than 19% to 32%. CS can be diagnosed with less but clinically sufficient certainty also if the histology is proved in an extracardiac tissue sample and clinical manifestations and findings at cardiac gadolinium-enhanced magnetic resonance imaging (Gd-MRI) or F-18-fluorodeoxyglucose positron emission tomography (F-18-FDG PET) are compatible with myocardial involvement. Since the first published cardiac F-18-FDG PET images in CS, with focally increased myocardial glucose uptake signaling inflammatory activity, PET has gained wide use in the initial diagnosis, assessment of disease activity, and monitoring of treatment response in CS. Moreover, whole-body PET can uncover hidden inflammatory foci in extracardiac organs, thus suggesting targets for diagnostic tissue biopsies. We set out to review our nationwide CS registry for details of the use of F-18-FDG PET in the detection of CS. In the present work, we focus on extracardiac PET findings and show their utility in the diagnosis of CS.
Methods
The registry of Myocardial Inflammatory Diseases in Finland has collected all patients diagnosed in our country with clinically manifest CS since the turn of 1990s. The criteria of CS required for inclusion in the registry have been detailed in our earlier reports. In brief, either myocardial or extracardiac histology of noncaseating granulomatous inflammation has been mandatory in addition to clinical manifestations and findings at cardiac imaging compatible with CS. From this database, we identified 72 patients with CS who had undergone F-18-FDG PET from 2005 to early 2013 as part of their diagnostic assessment after admission. Of them, 4 patients were excluded because of technically inadequate PET studies. The remaining 68 patients constitute the present study population. Their hospital charts were scrutinized in retrospect for demographics, cardiac signs and symptoms, laboratory tests, imaging studies, and diagnostic biopsies, and the data pertinent to the present work were collected for analysis. The study was performed according to the principles of the Declaration of Helsinki. The Ethics Committee for the Department of Medicine, Helsinki University Central Hospital, approved the study protocol, and the Myocardial Inflammatory Diseases in Finland registry study has been approved by the national ethical review board (STM/1219/2009). A proportion of the CS population (25 of the 68 patients) has been reported earlier.
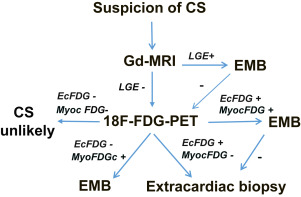
During the period covered by our work, patients presenting with etiologically unknown high-grade atrioventricular conduction disturbance, ventricular tachyarrhythmia, or heart failure were initially examined clinically and with routine laboratory tests, chest x-rays, and echocardiography. Once ischemic heart disease had been excluded, either clinically or at coronary angiography, patients usually underwent cardiac Gd-MRI unless it was contraindicated. If MRI was suggestive of myocardial inflammation by showing Gd late enhancement with or without edema or abnormal LV wall thinning or thickening, a right or left ventricular EMB was done aiming at the site of myocardial abnormalities identified in MRI. Cardiac F-18-FDG PET, usually complemented with whole-body image acquisition, was done (1) if MRI was contraindicated, (2) if the results of MRI were nonrevealing but suspicion of CS persisted, or (3) if the post-MRI EMB was nondiagnostic. If PET showed focally increased F-18-FDG uptake in the myocardium, EMB was again the next procedure. Extracardiac tissue biopsy was done, provided there was F-18-FDG accumulation outside the heart if (1) there was no abnormal myocardial F-18-FDG accumulation or (2) the post-PET EMB was negative. Figure 1 illustrates this diagnostic strategy.
Most F-18-FDG PET studies (58 of 68) were done at the Department of Nuclear Medicine, Helsinki University Central Hospital. The patients fasted for at least 12 hours before the examination, and blood glucose level had to be <7.0 mmol/L before the study. After an intravenous injection of F-18-FDG (303 ± 57 MBq), the uptake phase lasted at least 60 minutes. PET images were acquired with a Philips Gemini PET/computed tomographic (CT) scanner. For the whole-body images, scanning was performed from the proximal femoral region to the head. Cardiac image acquisition was done thereafter. Within 1 week from the PET/CT scan, a Tc-99m-tetrofosmin myocardial perfusion imaging at rest was performed. The details of these imaging methods have been described previously. Ten patients underwent PET in 3 other Finnish university hospitals according to their local routines. All PET studies, including the ones done elsewhere, were analyzed by an experienced nuclear medicine specialist (JS) at the core institution in Helsinki. Any pathologic uptake of F-18-FDG outside the heart was located and recorded, including the presence, number, and size of F-18-FDG–positive mediastinal lymph nodes (MLN). In cardiac PET, normal myocardial metabolism was defined as either complete suppression of F-18-FDG uptake or diffuse uptake without any areas of focally increased activity (i.e., without F-18-FDG “hot spot”). The Tc-99m-tetrofosmin myocardial perfusion at rest was compared with the F-18-FDG uptake in the 17-segment LV model, and each segment was visually classified as having either (1) normal perfusion and normal metabolism, (2) abnormal perfusion or abnormal metabolism (i.e., “hot spot”), or (3) abnormal perfusion and abnormal metabolism.
All statistical analyses were performed with the SPSS for Windows 19.0 Statistics (SPSS, Chicago, Illinois). The data are presented as mean ± SD for continuous variables and as absolute numbers or percentages for categorical variables, unless otherwise noted. Normal distribution and homogeneity of variance were checked before further analyses. Between-group comparisons for continuous variables were made with the Student’s 2-sided t test. Comparisons of discrete variables between groups were assessed with the chi-square test or Fisher’s exact test. All statistical tests were 2 tailed, and p <0.05 was regarded as statistically significant.
Results
The CS population comprised 47 women and 21 men with a mean age of 50 ± 9 years. Their main presenting cardiac manifestations were, in order of decreasing frequency, complete atrioventricular block (n = 37, 54%), sustained ventricular tachycardia (n = 18, 26%), congestive heart failure (n = 7, 10%), ventricular fibrillation (n = 4, 6%), and multiple ventricular premature beats (n = 2, 3%). An impaired LV function at echocardiography on admission (ejection fraction <50%) was found in 33 patients (49%), and Gd-MRI revealed pathologic LV wall late enhancement in 33 of the 43 patients studied (77%). Cardiac PET was abnormal in 62 of 68 patients. It showed both an F-18-FDG “hot spot” and a myocardial perfusion defect in 52 patients and either a perfusion defect or a “hot spot” in 10 patients. Of the 6 patients with normal cardiac F-18-FDG PET, 5 had pathologic late enhancement at Gd-MRI.
Cardiac and whole-body PET combined showed pathologically increased F-18-FDG uptake ( Figure 2 ) in MLN in totally 43 of the 68 CS patients (63%). Most of the PET-positive lymph nodes were located in the right upper and lower paratracheal regions, subcarinally and in the subaortic and para-aortic areas. Their number ranged from 2 to 14 per patient (mean 4), and their size averaged 1.2 ± 0.3 cm. Table 1 gives a complete list of sites of pathologic extracardiac F-18-FDG uptake in the 57 patients who underwent whole-body PET imaging. The data show that although 18 of 57 patients (32%) had F-18-FDG accumulation outside mediastinum, all except 1 of them also had parallel MLN involvement. None of the 6 patients with normal cardiac PET had abnormal extracardiac F-18-FDG activity, indicating that these 6 studies (9% of all) were completely false negative.




