Fig. 16.1
Shunting of left femoral artery and vein in a polytraumatized patient to restore distal perfusion in a damage control setting
Temporary shunts have a variety of applications in the management of extremity vascular trauma. In patients with associated fractures or dislocations, the placement of a temporary vascular shunt allows the orthopedic team to manipulate and fixate the injured extremity without concern for disrupting a vascular anastomosis. The shunt also minimizes ischemic time by providing perfusion during the orthopedic part of the procedure and during vein harvest and preparation. After the reduction and/or fixation, definitive vascular repair can be performed on a stable musculoskeletal platform. Likewise, in hemodynamically unstable patients, placement of a temporary vascular shunt permits more rapid restoration of extremity perfusion mitigating some of the ischemic risks associated with a prolonged vascular repair procedure.
Several vascular shunts are available for utilization. Argyle shunts commonly come in a single container with a variety of sizes making useful for a variety of vessels. Other commercially available conduits, including Javid, Sundt, and Pruitt-Inahara shunts, can also be effective depending upon the size of the vessel and the location of the injury. In extreme circumstances, even intravenous tubing or other soft sterile cylindrical structures can be utilized to temporarily restore perfusion.
The technique of temporary vascular shunt placement requires proximal and distal control of the injured vessel. An embolectomy through the disrupted segment of the artery should then be performed to remove proximal and distal clot and allow temporary restoration of perfusion. Flushing of the vessel with ample quantities of heparinized saline can clear any residual thrombus. Local infusion of heparinized saline rarely contributes to systemic coagulopathy, but should be used cautiously in patients at significant risk for coagulopathy or bleeding from brain, abdominal, or other injuries. Systemic heparinization for isolated extremity injuries without bleeding risk may also be considered; however, the effect of this on the patency of the temporary shunt or the subsequent formal vascular reconstruction has not been well established. Clinical experience, combined with data from animal models of hemorrhage and shock, suggests that temporary vascular shunts placed in the larger, more proximal extremity vessels have high rates of prolonged patency without utilization of systemic anticoagulation [35]. Once positioned, the shunt should be secured both proximally and distally to mitigate the risk of dislodgment during orthopedic manipulation or transportation.
The Stable Patient: Vascular Management Considerations
Patients who are hemodynamically normal can usually tolerate a definitive vascular repair at the time of their initial operation. All repairs begin by gaining vascular control proximal and distal to the site of injury. Once this fundamental surgical tenet has been fulfilled, the injury can be more clearly identified and characterized noting the quality of the injured arterial wall edges. Primary repair may be possible for discrete arterial lacerations with clean, viable injury margins as observed in some stab wounds. Debriding the vessel edges back to healthy, viable tissue is critically important to ensure the integrity of a suture repair or subsequent anastomosis. Because the external appearance of an injured artery may not reflect the extent of intraluminal injury or dissection, adequate debridement requires visualizing the arterial lumen. After debridement, short segments of vessel loss (1–2 cm) may be amenable to mobilization and tension-free primary repair. Longer segments of vessel loss, or the suggestion of tension after mobilization, mandate a vascular interposition graft to restore perfusion.
The choice of conduit, either native vein or synthetic graft, depends on several factors [36]. The diameter of the injured vessel, the degree of wound contamination, and the availability of autologous vein conduit should all be considered. For the majority of injuries, reversed great saphenous vein is the conduit of choice for repair. Although the topic has not been well studied, the preferential use of saphenous vein graft over polytetrafluoroethylene (PTFE) or other artificial materials [37] decreases the incidence of infectious and thrombotic complications associated with the prosthetic grafts. Synthetic grafts provide a reasonable alternative if the great saphenous vein is too small or not available due to injury burden.
The consistent anatomic location of the proximal great saphenous vein makes it easy to harvest for use as a vascular conduit. The incision to expose the vessel begins medial to the femoral pulse, just inferior to the inguinal crease, and travels distally along the anteromedial thigh. It is useful to first identify the saphenofemoral junction in the proximal thigh and then carry the dissection distally to obtain the length of venous conduit required for repair. Isolating the vein circumferentially with a vessel loop can speed the dissection with inadvertently damaging the vein by directly manipulating it. Most surgeons harvest the great saphenous vein from the uninjured (contralateral) lower extremity. Although its clinical importance has not been well established, this practice guards against using a vein that could harbor an unrecognized injury or intimal disruption. Using the contralateral vein also avoids contributing to venous hypertension in the injured limb by preserving its great saphenous vein. In theory, maintaining maximal venous drainage lowers the risk of developing compartment syndrome in the distal injured extremity. The graft should be placed in a reversed configuration to allow unimpeded flow in the direction of the valves. Using the marking pen to draw a series of lines on one side of the vein can help maintain its orientation and avoid twisting the conduit which cuts off flow and causes early thrombosis.
Once a conduit has been selected and properly oriented for interposition, both proximal and distal ends must be sutured into position using nonabsorbable suture on a tapered needle. The size of suture will be dictated by the vessel size, but for extremity injuries, 5-0 or 6-0 Prolene are common choices. Several specific nuances of suturing have been described, including parachute, triangulation, and a simple running suture [38]. All techniques effectively achieve a high-quality anastomosis if properly performed, and the surgeon should use the technique with which he or she is most familiar and facile. Prior to securing the final suture, retrograde and antegrade flushing should be performed by briefly releasing and reapplying the proximal and distal clamps. The anastomosis is then flooded with heparinized saline in an effort to definitively clear the lumen prior to restoration of flow.
At the completion of the repair, distal arterial flow should be assessed by pulse exam and Doppler assessment. Any deficiencies should raise concern for an issue with the graft or anastamosis, either technical error or thromboembolic occlusion. The pulse evaluation should be tempered by the fact that young patients are prone to vascular spasm and peripheral vasoconstriction in the setting of trauma and vessel manipulation. While vasospasm may be ameliorated intraoperatively using intraluminal vasodilators (e.g., low-dose nitroglycerine or papaverine), warming and resuscitation are often required to alleviate this otherwise normal response. A completion arteriogram can be performed by injecting contrast directly into the bypass conduit via a small gauge angiocatheter. This imaging study can confirm the patency of the bypass, distal anastomosis, and outflow. Frequent clinical reevaluation of the extremity in the form of postoperative vascular checks should be initiated and concerns for bypass failure mandate further imaging or re-exploration.
The proven safety and efficacy of endovascular therapy in elective vascular procedures triggered an interest in expanding these techniques to the treatment of vascular trauma and vessel injury [39, 40]. In many cases, endovascular technologies can be seamlessly integrated into the management of vascular extremity trauma. Digital subtraction angiography remains an important diagnostic tool for vascular injury, and modern sheaths and catheters allow select injuries to be treated through the same access site established for DSA with little delay or need for additional manipulation. In hybrid approaches to repair, occlusive endovascular balloons can be utilized to obtain proximal vascular control and decrease blood loss during subsequent open repair. For select injuries, endovascular stent grafting may even preclude the need for open surgery by providing intraluminal coverage of the injured vessel.
Although the role of endovascular technologies in vascular trauma has not been clearly defined, experience with these approaches continues to grow and early results have been encouraging [41–43]. Determining the patency and natural history of endovascular stent grafts placed in young trauma patients requires long-term studies which have not been conducted yet. Despite a lack of long-term follow-up, endovascular therapy will play an increasingly prominent role in vascular extremity trauma. Establishing a multidisciplinary team for the treatment of vascular injuries, including providers with endovascular skills, will help in making treatment decisions for these patients [43].
Considerations for Exposure of Specific Extremity Vascular Injuries
General Principles
Surgical access for vascular injuries should adhere to the basic surgical principle of proximal and distal control and repair of what is in the middle. Accordingly, the initial incision should be amenable to proximal or distal extension if necessary to improve visibility outside the zone of injury. Particularly when a hematoma is present, dissecting back to normal tissue planes frequently improves orientation and visualization allowing more rapid control above and below the injury.
Axillary Artery
Axillary artery exposure begins with an infraclavicular incision that parallels the clavicle and is capable of distal extension onto the arm. The next step requires division of the pectoralis major and minor muscles in sequence. Separating the muscles of the pectoralis major, with the “grain” of the muscle fibers, reveals the deeper pectoralis minor muscle. This muscle can be divided over a clamp just distal to its origin on the coracoid process to expose the underlying axillary artery.
Brachial Artery
The proximal brachial artery is most readily located via an incision on the medial upper arm in the groove between the biceps and the triceps muscles. In the setting of a large hematoma, care must be taken to avoid injury to the neurovascular bundle, which may be surprisingly superficial. The first structure encountered in the bundle is the median nerve, which should be preserved and protected if it is uninjured.
Distal brachial artery exposure often requires exposure at the antecubital fossa. A sigmoid incision that has its transverse component across the antecubital skin crease is most commonly used. At this location, the artery can be isolated just deep to the biceps tendon. For the sake of access, this tendon can be divided and tagged for later reconstruction. The sigmoid incision can be carried superiorly along the medial aspect of the upper arm to facilitate proximal control or distally for exposure of the brachial artery bifurcation.
Radial and Ulnar Arteries
The bifurcation of the brachial artery can be exposed using a sigmoid incision crossing the antecubital fossa. In the superficial tissues, the median cubital and basilic veins should be identified and preserved if possible. The medial antebrachial cutaneous nerve, which courses along the basilic vein, should also be protected from injury. Opening the deep brachial fascia at this location will reveal the neurovascular bundle, where the median nerve is the first structure encountered. The median nerve can be gently retracted medially, revealing the underlying brachial artery and the more lateral brachial vein (Fig. 16.2).
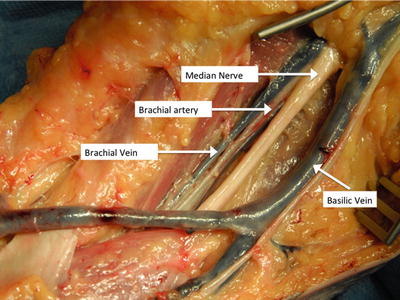

Fig. 16.2
Dissection of the brachial artery in the proximal forearm. The deep brachial fascia has been removed to show vascular structures both superficial and deep to this layer
The bicipital aponeurosis, which overlies the bicipital tendon, is the gateway to the bifurcation of the brachial artery and the proximal radial and ulnar arteries. Division of the bicipital aponeurosis allows exposure of the brachial bifurcation. Distal dissection provides exposure of the ulnar or radial arteries toward the wrist. If either the ulnar or radial artery is injured, an intraoperative Allen’s test will be established if single-vessel perfusion of the hand will be adequate for hand viability. In the majority of instances, individual injuries to either of these vessels in isolation can be safely managed with ligation alone.
Femoral Artery
In proximal femoral artery injuries, particularly in unstable patients with complex injury patterns potentially involving the abdomen, the decision to attain proximal vascular control in the abdomen should be considered. If the injury is truly isolated to the groin, however, two options for rapid control are available. The first, and most commonly utilized, is that of a vertical incision over the femoral triangle. This incision can then be extended superiorly with division of the inguinal ligament to establish definitive proximal control. Alternatively, an oblique incision superior and parallel to the inguinal ligament (similar to a renal transplant incision) can expose the external iliac vessels in an extraperitoneal location.
Effective dissection of the groin requires the ability to quickly establish wide exposure and identify and control the common, superficial, and deep femoral arteries and their accompanying veins. The lateral circumflex iliac vein crosses the deep femoral artery very close to its origin and can be injured during looped control of the deep femoral artery. This vein can be isolated, suture ligated, and divided to facilitate adequate exposure.
A medial thigh incision, either as an initial incision if the injury is known or as an extension of a proximal incision, provides the most convenient and facile access to the distal superficial femoral artery, femoral vein, and the proximal popliteal artery. The sartorius muscle, when retracted upward or downward, facilitates identification and opening of Hunter’s canal. Controlled dissection can identify and avoid unnecessary injury to the saphenous vein and its accompanying nerve.
Popliteal Artery
Adequate exposure of the popliteal artery can be challenging. Injuries at the popliteal bifurcation are best exposed by a generous incision on the proximal medial calf. Often, adjacent nerves and veins have also sustained injury making the dissection more difficult. More proximal popliteal artery injuries are better accessed by extending the medial thigh incision described above. The great saphenous vein at this location can be superficially located and susceptible to injury. Preserving this vein and protecting it from injury during surgical exposure may promote improved collateral venous outflow should the popliteal vein require ligation due to injury.
The presence of a large hematoma can make it difficult to quickly identify and isolate the injured vessels. Some guidance can be gained from the general principle that major neurovascular bundles of the lower extremities are always located immediately behind the bone. Accordingly, the distal superficial femoral artery and the proximal popliteal artery will be found directly behind the femur, and the popliteal bifurcation and tibioperoneal trunk will be found immediately behind the tibia. At either level, the accompanying vein is often encountered before the artery during dissection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


