Extracorporeal Membrane Oxygenation
Michael S. Mulvihill
Jacob N. Schroder
Mani A. Daneshmand
Introduction
Extracorporeal membrane oxygenation (ECMO) is oftentimes the only mode of salvage for decompensated heart and/or lung failure patients. ECMO therapy utilizes a continuous extracorporeal circuit to directly oxygenate and remove carbon dioxide from the blood. ECMO is a direct extension of the principles of cardiopulmonary bypass (CPB), modified to provide longer durations of support. ECMO requires decreased levels of systemic anticoagulation, and results overall in decreased coagulopathy and inflammatory response in comparison to CPB.
The ECMO circuit consists of the following primary features: (1) A hollow-fiber membrane oxygenator that permits gas exchange by way of direct blood gas interface providing simple control of both oxygenation and ventilation. (2) A heat exchanger that permits appropriate warming or cooling as blood circulates through the oxygenator past a countercurrent water supply. (3) A centrifugal pump which provides circulation of the blood. Flow through centrifugal pumps is afterload dependent, therefore a flow meter is required to quantify pump output as rotational speed alone does not accurately reflect blood flow.
Flow through the ECMO circuit is dictated by cannula and circuit tubing size. Blood flow into the ECMO circuit is typically established with either single- or dual-stage venous cannulas and blood return is established with either arterial cannula or Dacron “chimney” grafts. By Poiseuille law, flow through the circuit is inversely proportional to the fourth power of the diameter of the cannulas and tubing.
Depending on implementation and configuration of the circuit, ECMO can be deployed as a medium-duration therapy for patients with respiratory failure refractory to conventional oxygenation strategies and for patients in cardiogenic shock.
Indications for Veno-Venous Extracorporeal Membrane Oxygenation (VV-ECMO)
VV-ECMO may be initiated in patients with profound gas exchange abnormalities when positive pressure ventilation cannot maintain adequate oxygenation or ventilation.
ECMO should be considered in patients with a high risk of mortality from respiratory failure, commonly associated with PaO2/FiO2 ratios under 150 despite maximal conventional therapy.
ECMO should be considered in patients with a high risk of mortality from respiratory failure, commonly associated with PaO2/FiO2 ratios under 150 despite maximal conventional therapy.
In addition to hypoxic respiratory failure, patients with ongoing CO2 retention during a period of mechanical ventilation with high peak plateau pressures >30 cm H2O may benefit from ECMO support. Patients with severe air leaks, significant pulmonary embolism, or obstructed airways that have failed more conservative measures should also be evaluated for VV-ECMO support.
Indications for Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO)
VA-ECMO support should be considered in patients with refractory cardiogenic shock with or without end-organ dysfunction. This clinical scenario may present following cardiac arrest, cardiotomy with an inability to separate from CPB, acute myocardial infarction, myocarditis, postpartum cardiomyopathy, or decompensated chronic heart failure. Patients considered for VA-ECMO may be simultaneously evaluated for other forms of temporary mechanical support, or may have failed less invasive support. ECMO affords this patient population more support than intraaortic balloon pump (IABP) or temporary ventricular assist device (VAD). Peripheral cannulation of VA-ECMO permits rapid restoration of biventricular support, oxygenation, and circulation in the ICU or ED setting during the active resuscitation period. VA-ECMO should be considered as a bridge therapy to either recovery, cardiac transplantation, or durable VAD.
Contraindications to Veno-Venous Extracorporeal Membrane Oxygenation (VV-ECMO)
Current guidelines suggest that no conditions be considered absolute contraindications to VV-ECMO, therefore treatment decisions must be individualized based on assessment of risks and benefits to ECMO support. To this ends, a series of negative prognostic indicators should be considered before initiating VV-ECMO support.
Conditions such as recent central nervous system (CNS) hemorrhage that preclude the use of systemic anticoagulation should be considered a relative contraindication. Most centers require systemic anticoagulation, even for VV-ECMO. This can safely be paused in the setting of hemorrhage. In selected patients, with persistent bleeding, anticoagulation can be stopped entirely, albeit with an increased risk of circuit thrombosis and pulmonary embolus. VV-ECMO is also contraindicated in patients with severe or irreversible end-organ dysfunction that would be expected to limit the overall benefit of ECMO support. Finally, long durations (>7 days) of high ventilatory support, such as high-pressure ventilation (end-inspiratory plateau pressure >30 cm water) or fraction of inspired oxygen greater than 80%, should be considered negative prognostic indicators but not prohibitive contraindications in isolation.
Contraindications to Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO)
Since VA-ECMO increases mean arterial blood pressure (MAP) and LV afterload, the only absolute contraindication to this therapy is severe aortic regurgitation (AR). VA-ECMO with severe AR could result in massive LV distention and severe hydrostatic pulmonary edema. VA-ECMO is commonly utilized as bridge to either durable LVAD or transplant for cardiogenic shock patients. As such, in patients deemed to have unrecoverable heart function who are known to not be candidates for transplantation or LVAD support, VA-ECMO therapy is contraindicated. In addition, chronic organ dysfunction and prolonged cardiopulmonary resuscitation (>1 hour) without adequate tissue perfusion are highly negative prognostic signs, and ECMO therapy would be relatively contraindicated. As with VV-ECMO, patients with recent CNS trauma and evidence of hemorrhage for whom systemic therapeutic anticoagulation is contraindicated,
may be inappropriate candidates for ECMO therapy. However, we have supported patients with VA-ECMO without anticoagulation up to 48 hours without thrombotic complication.
may be inappropriate candidates for ECMO therapy. However, we have supported patients with VA-ECMO without anticoagulation up to 48 hours without thrombotic complication.
ECMO should be deployed by clinicians with training and experience in the initiation, maintenance, and discontinuation of therapy. ECMO requires the availability of a perfusionist to oversee and manage the circuit and central unit controller. Peripheral ECMO can be deployed in the intensive care unit (ICU), emergency department (ED), or operating room (OR). Central cannulation for ECMO is a major surgical procedure and is best performed in the OR. We recommend a team-based approach to deployment of ECMO, especially at the bedside. In our practice, bedside deployments are performed by a multidisciplinary team consisting of OR nursing, ICU nursing, surgeons, perfusionists, respiratory therapists, and critical care.
Positioning
The patient is positioned supine. Peripheral VV-ECMO cannulation can be achieved either with a single dual–lumen bicaval cannula (Avalon Elite cannula, Maquet) from the internal jugular (IJ) vein or with two single-lumen cannulas (either bilateral femoral veins or one femoral vein and the IJ). Peripheral VA-ECMO is typically achieved with isolated groin access or axillary artery access. All of these strategies (as well as central cannulation) can be performed with the patient supine. In adult patients, fluoroscopic guidance is required for the insertion of the Avalon Elite cannula. Some centers may utilize echocardiography for pediatric Avalon Elite cannulation. While fluoroscopy is recommended for peripheral VA-ECMO cannulation, it is not mandatory.
Technique: VV-ECMO
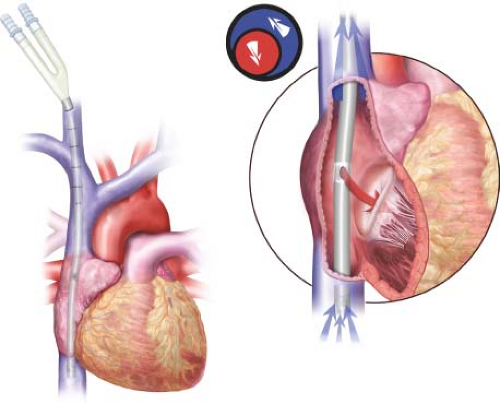
The preferred strategy for VV-ECMO cannulation is single-site, bicaval, double-lumen cannulation with the Avalon Elite cannula. The cannula is sized based on height and weight of the patient. The largest diameter cannula that can safely fit in the IJ and SVC is selected to provide optimal support. Typically, an average-sized adult can accommodate either a 27- or 31-Fr cannula. Patients with instrumentation of the SVC (like pacing wires or heart transplant recipients) may require smaller cannulas. Percutaneous access of the IJ is obtained using ultrasound guidance. The guidewire is directed to the inferior vena cava and “parked” in the iliac vein. Systemic anticoagulation is achieved with 50 to 100 units per kg of IV heparin. The tract is serially dilated and the cannula is inserted until the most proximal port is aligned with the confluence of the innominate vein and SVC while the middle “return” port is firmly in the body of the right atrium and the distal port is in the IVC (Fig. 38.1). The lumens are de-aired and connected to the ECMO circuit. The cannula is then secured to the skin with suture.
The dual-lumen nature of the cannula permits simultaneous venous drainage of blood from both the superior and inferior vena cava and return into the right atrium. The use of a single dual–lumen catheter permits central access through a single site and minimizes recirculation of blood (improving the efficiency of the ECMO circuit). Should a dual-lumen cannula not be available, two single-lumen cannula can be placed using Seldinger techniques. It is important that the inflow into the pump be from a single stage cannula in the IVC and the outflow of the pump be in the right atrium to minimize recirculation and maximize blood flow.
Technique: VA-ECMO
Femoral Cannulation
The femoral vessels are identified by landmarks or by ultrasound guidance. The femoral vessels are accessed percutaneously in an aseptic manner. If fluoroscopy is available, the venous cannula guidewire is directed and “parked” in the SVC and the arterial cannula guidewire is “parked” in the descending thoracic aorta. In the event of failure of a percutaneous approach, a surgical cut-down of the femoral vessels should be performed. Once the patient is systemically anticoagulated with 50 to 100 units per kg of IV heparin, the tracts are serially dilated and the cannulas introduced. If a multistage femoral venous cannula is used, the tip of the cannula is advanced into the orifice of the SVC, otherwise, the tip should remain in the mid portion of the right atrium. The femoral arterial cannula is advanced into the iliac artery. Placement of a 4- to 6-Fr distal perfusion catheter into the ipsilateral superficial femoral artery should be considered to minimize the risk of distal limb ischemia. Lines are then de-aired and connected to the ECMO circuit. The cannulas are secured to the skin with suture. ECMO is initiated. As a general rule of thumb, the largest peripheral venous cannula that can be safely placed should be used to ensure adequate drainage. Conversely, the smallest arterial cannula that can provide sufficient flow to maintain tissue oxygen delivery should be used to prevent limb ischemia, cannulation site bleeding, and other traumatic complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree