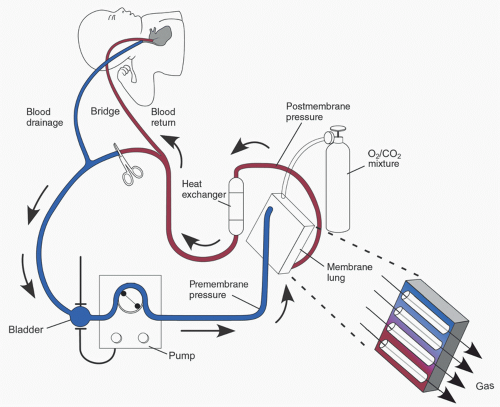
The typical ECMO circuit consists of tubing, a mechanical blood pump, membrane lung (oxygenator), cannulas for blood drainage (venous) and return (arterial), reservoir (bladder), heat exchanger, and pressure, flow, and oxygen saturation monitors (
1,
14).
Figure 31.1 is an illustration of a typical ECMO circuit, with the circuit components discussed in detail later in the chapter. To provide cardiorespiratory support, blood is drained from the venous circulation into the ECMO circuit. A pump then propels blood through the membrane oxygenator for gas exchange. The oxygenated blood is warmed to the desired body temperature and returned to the patient via the arterial cannula.
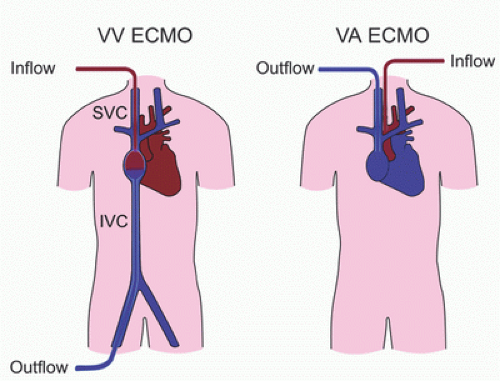
ECMO is used in two distinct support modes: venoarterial ECMO (VA ECMO) and veno-venous ECMO (VV ECMO) (
1,
2,
15,
16). The differences between the two modes are listed in
Table 31.1 and discussed below in detail.
Veno-Arterial ECMO
In this mode, blood from the venous circulation is drained into the ECMO circuit, pumped through the oxygenator for gas exchange, and the oxygenated blood from the circuit is returned to the arterial circulation (
1,
2,
15,
16). In this configuration, ECMO provides both cardiac and respiratory support (
Figs. 31.1,
31.2).
Preload to the heart is decreased during VA ECMO because blood is drained from the venous circulation into the ECMO circuit (
17,
18,
19). Decreased preload reduces myocardial contractility, left ventricular stroke volume, and pulse pressure. When the venous circulation is completely drained into the ECMO circuit, nonpulsatile flow ensues. However, in myocardial disease, decreased myocardial contractility and loss of pulsatile flow can occur prior to complete venous drainage, as injured or diseased myocardium may require higher
end-diastolic pressure and volume for myocardial contractility. When myocardial injury or disease improves, myocardial contractility returns, left ventricular ejection increases, and pulsatile flow resumes. Because decreased preload reduces contractile function of the ventricle, status of myocardial preload should be taken into account when evaluating myocardial contractility during ECMO.
Afterload to the left ventricle (LV) is increased during ECMO support (
17,
20,
21). Increased afterload may be due to systemic vasoconstriction from sympathetic activation and the stress response to critical illness, use of inotrope and vasoconstrictor infusions, and arterial flow from the ECMO circuit into the aorta. Increased afterload increases LV end-diastolic pressure (LV-EDP), LV wall stress, and left atrial (LA) pressure (
22,
23,
24,
25,
26). The manifestation of LA hypertension during VA ECMO is severe pulmonary edema, pulmonary hemorrhage, and “white-out” of the lung fields on chest X-ray (
Fig. 31.3). Furthermore, increased LV-EDP and wall stress can impair myocardial recovery. Because the LV is not directly drained during VA ECMO, left heart decompression may be required in some patients supported with VA ECMO to lower LA pressure and reduce pulmonary edema and hemorrhage. Left heart decompression may also reduce LV wall stress and thereby promote myocardial recovery. Left heart decompression can be achieved in the interventional cardiac catheterization laboratory by creating an atrial communication (balloon atrial septostomy), or by draining the LA into the venous limb of the ECMO circuit using a drainage cannula (LA venting cannula).
Cardiac output during VA ECMO is a combination of the ECMO flow and native cardiac ejection. ECMO flow and systemic vascular resistance (SVR) determine mean arterial blood pressure (MAP) (
18). In VA ECMO without native LV ejection, oxygenated blood from ECMO arterial return flows retrograde in the ascending aorta and aortic arch to provide coronary blood flow. However, in those patients with native LV ejection, coronary blood flow is antegrade from native LV ejection. Native LV ejection contains blood that is not drained into the ECMO circuit from the right atrium (RA) but ejected into the pulmonary circulation by the right ventricle and returned to the left heart. Mechanical ventilation with appropriate inspired oxygen concentration (Fio
2) is required to oxygenate the blood transiting the pulmonary circulation and returning to the left heart in order to deliver oxygen to the myocardium. In an animal model of VA ECMO, Shen et al. (
27) have shown that myocardial perfusion with oxygenated blood, provided by mechanical ventilation with an appropriate Fio
2, was associated with improved myocardial recovery.
Veno-Venous ECMO
In VV ECMO, venous blood is drained from the venous circulation into the ECMO circuit, pumped through the oxygenator, and returned to the RA (
Fig. 31.2) (
1,
15,
16,
28). Oxygenated blood returned to the RA from the ECMO circuit enters the RV through the tricuspid valve (TV) and is ejected into the pulmonary circulation (
Fig. 31.4). The oxygenated blood transits the pulmonary circulation to the left heart and is ejected by the LV into the systemic circulation. Thus,
VV ECMO does not provide cardiac support and depends on native cardiac function to maintain cardiac output.
Systemic oxygen saturation (Sao
2) depends on the proportion of oxygenated blood entering the right ventricle (RV)
(
28,
29). Therefore, positioning the return limb of the venous cannula such that oxygenated blood from the ECMO circuit returning to the RA is directed toward the TV is crucial (
Fig. 31.4).
Recirculation of some oxygenated blood returned to the RA back into the venous drainage is common. However, recirculation of significant amounts of oxygenated blood results in reduced Sao
2. Recirculation can be assessed by comparing the partial pressure of oxygen in the arterial (Pao
2) and venous (Pvo
2) limbs of the VV ECMO circuit. A Pvo
2 <20% of the Pao
2 is acceptable and does not impair the efficiency of VV ECMO (
30). Decreased Sao
2 during VV ECMO should prompt the evaluation of cannula position to ensure that oxygenated blood returned to the RA is directed toward the TV. Other causes of low Sao
2 during VV ECMO include hypovolemia, RV dysfunction, increased pulmonary vascular resistance (PVR), and developing oxygenator failure. These issues should be promptly investigated and treated.
Choosing an ECMO Mode
Patients with severe cardiac dysfunction due either to primary cardiac disease or secondary to other diseases (e.g., sepsis) require VA ECMO (
1,
14). VV ECMO is used to support children with respiratory failure with adequate cardiac function and output. Children with heart disease presenting with pure respiratory failure can be successfully managed with VV ECMO (
31,
32). Some patients with hypoxemic respiratory failure may have cardiac dysfunction secondary to hypoxemia. Restoration of systemic oxygenation with VV ECMO in these patients can improve cardiac function by correcting respiratory acidosis, reducing PVR, and enhancing myocardial oxygenation. In these patients, an initial trial of VV ECMO can help avoid the need for VA ECMO.