Exercise Screening and Sports Participation
Julie A. Brothers
Paul Stephens Jr.
Stephen M. Paridon
Over the last 30 years, there has been a significant increase in the rates of overweight and obesity in children in developed countries. Concurrently, there has been a decline in physical activity in children and adolescents. The reasons for these trends are multiple and include a shift to a high-fructose diet, increased fat and processed food consumption, and increased sedentary activity such as watching television and video gaming. The result is a young population at risk for an epidemic of hypertension, type II diabetes, and early atherosclerotic coronary disease (1,2). Recent data on obesity trends in children with congenital heart disease (CHD) indicate their incidence of obesity is similar to that of the general pediatric population. The risks for complications of obesity appear to be at least as great in this population as in the general population (3,4). Given these trends, there is an essential need to promote routine vigorous physical activity in both the general pediatric population and, in particular, the CHD population.
Balanced with the need to promote physical activity as an essential part of a healthy lifestyle is the need to keep children and adolescents safe from the risk of sudden cardiac death (SCD) during physical activity. Although SCD is very rare in the pediatric and young adult population, congenital cardiac defects, either myopathies or structural abnormalities, as well as primary arrhythmias and channelopathies are the major causes of these events (5,6). SCD occurs very rarely in patients with known congenital cardiac disease. Much more commonly, it happens to those athletes not previously suspected of having cardiac abnormalities. There are few evidence-based recommendations for the screening of athletes for risks of SCD. Much controversy surrounds this topic. There are even fewer evidence-based data regarding the screening and participation for athletic activities for children and adolescents with known CHD. What information is available is restricted to adolescents and young adults, and, importantly, current recommendations are restricted to competitive sports. There is essentially no information on the safety or screening of preadolescents with CHD for physical activity. As well, there are almost no evidence-based guidelines for children and adolescents with CHD wanting to participate in leisure or recreational activities. Recently, the American Heart Association (AHA) published a scientific statement discussing the promotion of physical activity, to be distinguished from exercise, in children and young adults (7).
The goal of this chapter is to discuss the current state of knowledge for athletic screening and participation in children with CHD. It broadly discusses the current recommendations regarding physical activity promotion in the pediatric, adolescent, and young adult populations. It also discusses the differences and various types of physical activity such as those of daily living, leisure sports, and organized competitive athletics. As well, differences in physiologic requirements of various athletic activities and their associated risks are discussed. The current methods and recommendations for screening for SCD during physical activity are reviewed. Controversies regarding the type and timing of screening are addressed. Finally, what is known about the ability to participate in and the risks of athletic activities for the individual groups of congenital cardiac defects is discussed with emphasis on what is known about preathletic screening and recommendations for activities of daily living, leisure and recreational athletics, and participation in competitive sports.
Physical Activity and Exercise
Physical performance during exercise depends on the individual’s strength, endurance, and skill in performing a given athletic activity. These in turn may be influenced by a number of factors such as age, sex, height, weight, and especially cardiovascular conditioning. The ability to successfully and safely undertake a given athletic activity depends on the combination of these factors and the requirements of the activity.
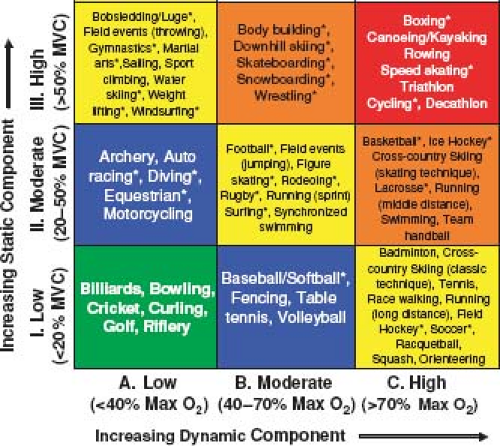
All athletic activities can be roughly broken down into their “static” and “dynamic” components (8). The static component is the amount of maximal voluntary contraction (MVC) of the exercising muscles required to perform the activity. This is what is traditionally considered isometric activity or the muscles working against resistance. Examples of activities requiring high static forces are weight lifting and wrestling. The cardiovascular effects of isometric activity depend on the intensity of the activity (e.g., the percentage of MVC required) and the size and number of the muscle groups involved in the activity. Contraction of muscles during isometric activity results in an increase in systemic vascular resistance (SVR) with a consequent rise in systolic, diastolic, and mean blood pressures. The degree of increase in these values depends on the size of the muscle group and the percentage of MVC achieved but may result in systolic blood pressures in excess of 300 mm Hg with heavy weight lifting. On the other hand, cardiac output and total body minute oxygen consumption (VO2) are relatively unchanged by brief severe static activity (9,10,11).
The dynamic component of exercise can be thought of as the activity that results in muscle contraction and body movement. These activities are usually repetitive and against low resistance (8,10). This is what is thought of as isotonic activity. An example of a highly isotonic activity is long-distance running. The cardiovascular effects of high dynamic activity are quite different from static activity. The metabolic demands of the exercising muscle are much greater. The VO2 may rise tenfold or more from resting values with high-dynamic activity. To meet this oxygen demand, cardiac output may rise fivefold or more in well-conditioned athletes. Although systolic blood pressure rises as the cardiac output increases, vasodilation of the vascular bed of the exercising muscles results in a significant drop in SVR with high-dynamic exercise. Thus, dynamic exercise results primarily in a volume load being placed on the heart as opposed to the pressure load that results from highly static activity.
In truth, there are no pure “static” or “dynamic” activities, and all athletic activities are to some degree, a combination of both types. Figure 10.1 shows the relative amounts of static and dynamic forces required for various types of competitive sports that were published as part of the 36th Bethesda Conference on Competitive Sports Participation in Athletes with Heart Disease (8). Increasing dynamic activity requires a higher VO2 while increasing static activity requires a higher percentage of MVC. There are sports such as rowing and cycling that require both high static and high dynamic components.
This figure has been widely published and used by clinicians to help make recommendations for activities for their patients with heart disease. It is important to remember that the values for this table refer only to competitive sports in adolescents and adults. The contents of this table have little or no relevance to competitive sports participation in the preadolescent population. Much of preadolescent competitive sport training focuses on learning basic skills and coordination. Strength and endurance training have very little or no place in competitive sports at this age. Any parent will tell you that soccer played by a group of 7-year-olds bears almost no relationship to soccer being played by a group of 17-year-olds. Understanding these differences is crucial since it impacts directly on the ability of athletes with CHD in these age groups to successfully and safely participate in the athletic activity.
Types of Physical Activity
Physical activities can be divided into three broad types of activities: (a) activities of daily living, (b) leisure and recreational sports, and (c) competitive sports. All patients with CHD participate in the first type of activity. Many also participate in one or both of the latter two types. Understanding the differences between these types of activities is important to being able to assess the capabilities and safety of individuals with CHD to perform these activities (12).
Activities of Daily Living
“Activities of daily living” is an inclusive term that encompasses all the physical activities required by an individual as part of his or her routine daily tasks. These requirements will vary greatly depending on the age of the individual as well as many other unique circumstances. There have been attempts to quantify the amount of physical activity that occurs during typical activities of daily living among different ages of children, adolescents, and young adults both with and without CHD (13). These studies used various types of motion detectors as well as recall questionnaires. The results of these studies were mixed, but generally indicated that children and adolescents with CHD perform significantly less physical activity as part of their daily routine (14,15,16). This difference was most pronounced in boys with CHD compared to their healthy peers (15,16). It is also worth noting that most children tend to overestimate the amount of physical activity they perform (17,18).
The reasons for this difference in physical activity associated with daily living among children with CHD are not clear. There is at least some evidence that this is due to activity restrictions that have been imposed by physicians, parents, and in some cases by the children themselves (19). This has been seen even in children with relatively trivial cardiac conditions. The consequence of these restrictions may well be quite significant. Recent studies of obesity trends in children with CHD are alarming (20). Obesity in this population mirrors that of the general pediatric population and occurs even in populations with otherwise excellent cardiac repair and normal or near normal exercise capacities (3,4,19). There is at least some evidence that the amount of obesity is related to daily amounts of physical activity (21).
Assessment of activities of daily living is even more important in the case of young adults with CHD (7). These activities will usually include those required for employment. The intensity of physical activity can obviously vary greatly from individual to individual depending on the nature of their employment. Although this may seem obvious, the little research available would suggest that patients and physicians largely ignore this aspect of care. Of concern is the finding that the most common reason patients do not seek information about appropriate level of physical activities is a mistaken belief that all activities are safe to perform (22).
Leisure and Recreational Sports
Leisure and recreational sports are physical activities that are engaged in without pressure to participate and are performed at the individual’s desire and comfort level. In short, they are activities that are undertaken without formal coaching. This definition encompasses a broad category of activities. Although there may be no formal coaching, some of these activities have significant organization and structure. Intramural sports at the high school or college level may often fall into this category. Less structured activities such as playground “pick-up” games as well as physical activities that may be undertaken by an individual such as cycling or jogging would also fall under this category of leisure or recreational sports. A careful history of these activities is an important part of the assessment of a patient with CHD. Clearly, the intensity with which these leisure activities are performed may vary widely with the age and circumstances in which they are undertaken (13). This also highlights the importance of understanding the difference and intensity of sports at a recreational level as opposed to a competitive level. For example, intramural flag football has little in common with competitive high school football. For this reason, it does not make sense to use the information in Figure 10.1 on the intensity of this sport to determine if it is safe for a patient with CHD to play at an intramural level.
Competitive Sports
Competitive sports are those that are generally organized, coached, and played at high skill levels. They often, but not always, require high-intensity physical activity (Fig. 10.1). The intensity with which an individual participates in competitive sports is influenced by their personal motivation as well as the outside influences of the coach, other team members, parents and other family members, as well as spectators. The end result is the potential for the individuals to push themselves to participate beyond the level they might otherwise choose to or which might be considered safe by their physician (12).
Training is also a significant part of competitive sports. The training for competitive sports may in many cases be of higher intensity than participation in the actual sport. For example, weight training and physical conditioning undertaken by high school athletes playing baseball may easily exceed the intensity of the activity they achieve in the actual game. When considering the safety of participation of an individual with CHD in a particular sport, the requirements of training must be considered equally with the sporting activity itself.
It is also important to remember, as stated earlier, that “competitive” does not necessarily mean the same thing for all ages. None of the factors that influence high levels of performance in adolescents and adults, such as coaching and spectators, are likely to have much of an effect on young children. Especially at early ages, children are very unlikely to perform beyond a level that they would otherwise choose to self-limit. As such, these “competitive” sports should be thought of more as activities to teach basic physical skills rather than true competitive athletics (12,23).
Promoting Physical Activities and Exercise in Patients with Congenital Heart Disease
Given the concerns of growing obesity and sedentary lifestyles in the population with CHD, what should be the recommendations for physical activity in this population? Regardless of age, in the vast majority of the population, the recommended level of 60 minutes of moderate to vigorous physical activities per day is probably appropriate. This level of activity corresponds to approximately 50% to 60% of maximal VO2 or 70% of maximal heart rate (24). As will be discussed later in this chapter, this is a level of physical activity that is often achieved in recreational activity or in many cases through competitive sports and is both safe and desirable for many individuals with simple congenital heart defects. Often with minor CHD, minimal formal testing may be required prior to individuals undertaking this level of activity; a thorough history and physical examination is likely all that is necessary. In more complex defects and usually following an operative repair, more formal studies including electrocardiogram (ECG), echocardiogram, and exercise testing may be indicated. The need and the rationalization for these tests will be discussed for the individual defects.
In patients with complex defects and residual cardiac dysfunction, studies from the adult heart failure literature suggest that they would still benefit from routine regular physical activity. However, these patients may need physical activity programs that are more specifically designed for their degree of cardiac fitness (25,26,27). In these circumstances, formal exercise testing with assessment of VO2, work rate, and heart rate is very useful in generating an exercise prescription. This can be used to instruct the patient in the types and intensity of activities that are both safe and beneficial. These prescriptions are usually based on what is referred to as FITT-factors. FITT is an acronym for Frequency, Intensity, Time and Type. All four factors should be included when generating an exercise prescription and address both activities with primarily dynamic and static components to assure optimal physical conditioning (28). This type of activity classification is used throughout this chapter in making recommendations for activities in individual congenital cardiac defects.
Preparticipation Screening for Undiagnosed Cardiac Conditions
Incidence of Sudden Cardiac Death
SCD in individuals younger than 35 years of age during or just after exercise is almost always attributable to structural or functional cardiac disease (29). In the United States, the most common structural cardiac cause of SCD is hypertrophic cardiomyopathy (HCM) with congenital coronary anomalies (usually those arising from the incorrect sinus with interarterial and intramural course) as the second most common cause. Other cardiac diseases, including myocarditis, other cardiomyopathies, electrical abnormalities (e.g., long QT syndrome, catecholaminergic polymorphic ventricular tachycardia, Brugada syndrome), Marfan syndrome, aortic valve disease, atherosclerotic coronary artery disease (CAD), and others make up the remainder of sudden death causes (6,29,30,31,32). Because there is no mandatory reporting system in the United States, the exact frequency of SCD is unknown. Published reports have relied on public media, catastrophic insurance claims, the U.S. National Registry of Sudden Death in Athletes, and electronic databases. From these, estimates of SCD range from 1:160,000 to 1:300,000 for competitive athletes aged 12 to 35 years (29,31,32). Indeed, mortality rates in collegiate, NCAA, athletes, appear to be somewhat higher when compared with high school athletes, possibly due to the greater amount of time training at a high level (6). These may underestimate the true incidence of SCD since without mandatory reporting, it is likely that some cases are missed. In United States military personnel, the rate of SCD is usually higher than those in high school and college athletes, likely due to very intense and prolonged physical exertion, often by people who were not physically prepared for the amount of strenuous activity. According to the most recent US Department of Defense registry, the SCD rate is 6.7/100,000 person-years in men and 1.4/100,000 person-years in women, with nearly half of the events occurring during or after physical exertion (33,34). In the Veneto region of Italy, prior to implementing a national screening program, the incidence of SCD was 1:28,000 for competitive athletes aged from 12 to 35 years. This is based on a mandatory registry of SCD in the region (35).
Risk of SCD differs based on gender, race, and activity. SCD occurs more often in males than females (30,31,34,36), which may be explained by the greater participation rates in males compared to females in competitive athletics. In the United States, African Americans are at greater risk than Caucasians for SCD (6,36). Participation in soccer and basketball carries the highest risk of SCD, but this may be explained by the popularity and higher participation rates in these sports compared to other activities (30).
Although debatable, many believe that regular training for athletic competition is associated with an increased risk of SCD in those athletes who have underlying occult cardiovascular disease compared to sedentary young individuals. In 2003, Corrado et al. reported the incidence of sudden death in the athletic and nonathletic young population (12 to 35 years) in the Veneto region of Italy. Young athletes participating in competitive sports had an estimated risk of sudden death approximately 2.5 times their non-competitive peers. They reported an incidence of sudden death of 2.3 (2.62 in the males and 1.07 in females) per 100,000 athletes per year from all causes and of 2.1 per 100,000 athletes per year from cardiovascular disease (37). As well, a recent nonforensic analysis of collegiate athletes found a frequency of 2.3/100,000 athlete-years for cardiovascular-related sudden death (9 athlete deaths per year over a 5-year study period) (38). This was a greater risk than was previously believed. However, Maron et al. examined multiple databases and autopsy reports evaluating the same population as the Harmon et al. study and also expanded the study period; they found a significantly lower rate of 1.2/100,000 athlete participation-years (4 to 6 deaths/year). In fact, they found that the risk of SCD in student athletes was relatively low with mortality rates similar to those found due to drug abuse and suicide (6).
Purpose of Preparticipation Screening
Why do we perform preparticipation screening? Is the purpose to prevent SCD or is it to identify those children with cardiovascular abnormalities that may place them at increased risk for SCD?
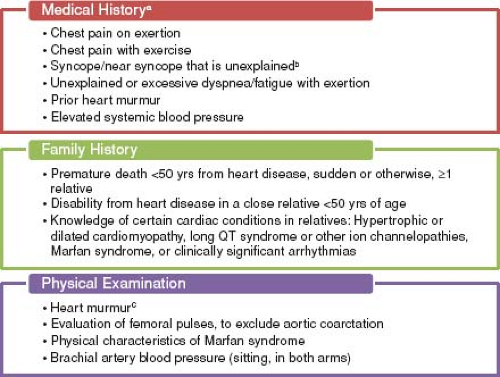
The answer appears to be the latter. The AHA states that preparticipation screening is the “systematic practice of medically evaluating large, general populations of athletes before participation in sports for the purpose of identifying (or raising suspicion of) abnormalities that could provoke disease progression or sudden death” (Fig. 10.2) (39). Similarly, according to the 36th Bethesda Conference Guidelines “the ultimate objective of pre-participation screening carried out in general populations of trained athletes is the recognition of ‘silent’ cardiovascular abnormalities that can progress or cause sudden cardiac death” (40). The American Academy of Pediatrics recently published guidelines for preparticipation screening, with the goal to “uncover conditions that might require further investigation or treatment” that would hinder the health and safety of the athlete (41). Thus, it appears that the main goal of screening is to discover underlying cardiovascular disease that has the potential for SCD. Based on estimates from several studies using noninvasive testing, approximately 1 in 500 young athletes may have an underlying cardiac condition that places them at increased risk of SCD (35,39,42,43,44,45). Many cardiovascular conditions, once identified, can be treated to help reduce the risk of SCD. Treatment may include activity restriction, pharmacotherapy, electrophysiology studies and procedures, implantable cardioversion defibrillator placement, and in some cases, surgical repair.
The answer appears to be the latter. The AHA states that preparticipation screening is the “systematic practice of medically evaluating large, general populations of athletes before participation in sports for the purpose of identifying (or raising suspicion of) abnormalities that could provoke disease progression or sudden death” (Fig. 10.2) (39). Similarly, according to the 36th Bethesda Conference Guidelines “the ultimate objective of pre-participation screening carried out in general populations of trained athletes is the recognition of ‘silent’ cardiovascular abnormalities that can progress or cause sudden cardiac death” (40). The American Academy of Pediatrics recently published guidelines for preparticipation screening, with the goal to “uncover conditions that might require further investigation or treatment” that would hinder the health and safety of the athlete (41). Thus, it appears that the main goal of screening is to discover underlying cardiovascular disease that has the potential for SCD. Based on estimates from several studies using noninvasive testing, approximately 1 in 500 young athletes may have an underlying cardiac condition that places them at increased risk of SCD (35,39,42,43,44,45). Many cardiovascular conditions, once identified, can be treated to help reduce the risk of SCD. Treatment may include activity restriction, pharmacotherapy, electrophysiology studies and procedures, implantable cardioversion defibrillator placement, and in some cases, surgical repair.
Current Preparticipation Screening Recommendations
Both the AHA and the European Society of Cardiology (ESC) agree that preparticipation screening of young athletes beginning at age 12 years is warranted; however, controversy exists between the United States and European recommendations on the inclusion of a 12-lead ECG as part of routine screening (39,46). The first consensus statement on preparticipation screening in the United States was published by the AHA in 1996 and was reaffirmed in 2007 (39,47). This statement recommended a detailed personal and family history and physical examination (Fig. 10.2). A 12-lead ECG was not included. One of the major issues with this approach is that many athletes with a predisposition to SCD are asymptomatic, with normal histories and physical examinations. Often, the first sign of an underlying cardiovascular condition is SCD in up to 60% to 80% of these athletes (30,48,49). The limited value of history and physical examination alone was noted in a retrospective analysis of 115 high school and collegiate athletes who died suddenly. The authors found that cardiovascular abnormalities were suspected by standard history and physical examination screening in only 3% of the examined athletes and screening led to the accurate diagnosis in only one athlete (30). In the United Kingdom, prospective studies using history and physical examination were not efficacious in identifying those with underlying cardiovascular conditions associated with SCD (43).
The current recommendations from the ESC, endorsed by the International Olympic Committee (IOC) and several professional sports organizations, include a 12-lead ECG in addition to the screening history and physical examination (46,50). For over 25 years, Italy has evaluated several million athletes annually, under a state-subsidized screening program (46). The evaluation is performed by specially trained physicians who work in centers dedicated to preparticipation screening of athletes. Screening starts at age 12 to 14 years and is repeated at least every 2 years (46). The protocol also incorporates guidelines for further investigations if any cardiovascular abnormality is found or suspected.
Benefits of Mandatory Preparticipation Electrocardiogram Screening
In the United States, the inclusion of additional noninvasive tests, such as the 12-lead ECG, to preparticipation screening of young athletes is highly debated (51,52,53,54,55). The European recommendations are based on several studies demonstrating increased sensitivity using ECG to detect occult cardiovascular pathology compared with history and physical examination alone (35,42,43,44,45).
Genetic cardiomyopathies are the most common cause of SCD in young athletes. In the United States, HCM is most common while in Italy, arrhythmogenic right ventricular cardiomyopathy (ARVC) predominates (29,35). While cardiomyopathy is definitively diagnosed with cardiac imaging, ECG screening can detect approximately 95% of those with HCM and 80% with ARVC (55,56). Corrado et al. (57) reported on a 17-year experience of preparticipation screening that included ECG in the Veneto region of Italy. During 1979 to 1996, a consecutive series of 33,735 young athletes was evaluated. Of these athletes, 1,058 were disqualified for medical
reasons and 621 (1.8%) because of the recognition of clinically relevant cardiovascular abnormalities. Among the athletes screened, 22 (0.07%) showed both clinical and echocardiographic evidence of HCM, which accounted for 3.5% of the cardiovascular causes for disqualification. The authors showed that, when compared to history and physical examination alone, ECG had 77% greater power to identify HCM. Similarly, a population-based study of 4,111 young adult athletes in the United States using ECG found a diagnosis of HCM in 7 (0.17%) by echocardiography, 5 of whom had an abnormal ECG (71%) (58). The Italian ECG-based preparticipation screening model has also been shown to have a high negative predictive value (99.98%) in excluding HCM in those young athletes who have normal ECG (59). This model was found to be effective in reducing rates of SCD from ARVC with a significant decline (84%) in incidence rates from the prescreening to the postimplementation of screening era (35).
reasons and 621 (1.8%) because of the recognition of clinically relevant cardiovascular abnormalities. Among the athletes screened, 22 (0.07%) showed both clinical and echocardiographic evidence of HCM, which accounted for 3.5% of the cardiovascular causes for disqualification. The authors showed that, when compared to history and physical examination alone, ECG had 77% greater power to identify HCM. Similarly, a population-based study of 4,111 young adult athletes in the United States using ECG found a diagnosis of HCM in 7 (0.17%) by echocardiography, 5 of whom had an abnormal ECG (71%) (58). The Italian ECG-based preparticipation screening model has also been shown to have a high negative predictive value (99.98%) in excluding HCM in those young athletes who have normal ECG (59). This model was found to be effective in reducing rates of SCD from ARVC with a significant decline (84%) in incidence rates from the prescreening to the postimplementation of screening era (35).
In 2006, Corrado et al. reported on a prospective observational analysis of 42,386 young athletes, evaluating the rate of SCD in those undergoing screening to those nonathletes who did not have preparticipation screening over more than 25 years. Results were compared for the era prior to use of ECG for preparticipation screening to the 25-year era with ECG screening. There was a 90% reduction in mortality in young athletes since the beginning of the screening period. The rate of SCD in nonathletes remained constant over the same period. The reduction appeared to be due to fewer cases of SCD from cardiomyopathies (mainly ARVC) and to increased identification of cardiomyopathies at time of screening (35). Recently, Wilson et al. evaluated 1,074 junior athletes (ages 10 to 27 years) and 1,646 physically active schoolchildren (age 14 to 20 years) in the United Kingdom using personal and family history, physical examination, and resting ECG. Nine athletes (0.3%) were found to have cardiovascular abnormalities predisposing them to SCD; all were detected by ECG alone, none had symptoms or family history of SCD (43).
Concerns about Mandatory Preparticipation Electrocardiogram Screening
In the United States, obstacles exist to implementation of an obligatory national screening of competitive athletes using ECG. Some of these obstacles include the large population of athletes to be screened (10 to 12 million) over a large land mass; diverse ethnic and racial population in the United States, which makes interpreting ECGs more difficult; lack of dedicated healthcare providers that would be necessary for a large screening program; and the recognition that it is impossible to absolutely eliminate the risks associated with competitive sports (60).
Another main concern regarding the use of ECG during preparticipation screening relates to the high false-positive rate. In the study by Corrado et al. (35), a 7% false-positive rate with 2% of athletes being disqualified was reported; only 0.2% of athletes were ultimately disqualified for potentially fatal cardiac conditions. This raises concerns regarding unnecessary further investigations and/or false disqualification of an athlete who is not actually at increased risk for SCD (61).
Several studies have been published utilizing strict ECG criteria when screening athletes to take into account the physiologic adaptation of heart structure and function in athletes (“athlete’s heart”) affecting the interpretation of ECGs. In the study by Wilson et al., the authors reported a false-positive rate of 3.7% using history, physical examination, and ECG with 1.9% false positives due to ECG alone (43). Hevia et al. (62) evaluated 1,220 amateur athletes in Spain using history, physical examination, and 12-lead ECG and found 6.14% of ECGs were abnormal with two cases of HCM diagnosed by ECG. There were 15 cases with positive criteria on history or physical examination, but none were found to have cardiac disease by echocardiography (1.2% false-positive rate). In 2007, Pelliccia et al. (63) described ECG abnormalities on 32,652 amateur athletes (median age 17 years) undergoing preparticipation screening in Italy and found 12% had abnormalities but only 40% of those abnormalities (4.8% of the population) required further diagnostic testing.
Two recent studies have further called into question the usefulness of 12-lead ECG as part of preparticipation screening of athletes. Maron et al. (64) compared sudden death rates in Minnesota, where ECG is not part of routine screening, to that of the Veneto region of Italy, where ECG screening is mandatory. Over a 23-year period, the SCD rate of high school athletes in Minnesota was 1.06 per 100,000 person-years and 1.87 per 100,000 person-years in Italy. These data demonstrated that the SCD rate was low in both locations and did not support a reduced mortality rate due to use of ECG in preparticipation screening. As well, Steinvil et al. (54) reported on the sudden death rate prior to and after mandatory preparticipation screening program in Israel. This screening consisted of medical history, physical examination, ECG, and exercise stress testing. The authors found no significant difference between the average yearly incidences of SCD (2.54 per 100,000 person-years) in the decade prior to mandatory screening compared to the decade after (2.66 events per 100,000 person-years). The authors also called into question the findings from Italy (35) from which the ESC based their current guidelines (46), noting that the Italian study only looked at the 2-year period preceding the enforcement of ECG screening and compared this to mortality rates 25 years later. Instead, it may be that there were abnormally high mortality rates in Italy in 1980 and 1981 (mandatory screening was enforced in 1982) but if they had looked prior to those dates, they may have found great variability in the SCD rates, as was found in Israel (35,54).
As well, as with any screening tool, ECG does not detect all conditions that predispose an athlete to SCD. In particular, congenital coronary anomalies and premature atherosclerotic CAD cannot be identified with ECG alone. These cause up to 20% of SCD in young athletes in the United States (30). There is also a small percentage of people with HCM who have a normal ECG; however, it is believed that these may represent a milder phenotype and may have a lower risk for cardiac-related sudden death (65).
Electrocardiogram Analysis
The evaluation of studies looking at false-positive and false-negative rates for ECG screening may be strongly affected by what is defined as normal and abnormal in an individual study. There are physiologic changes in structure and function that are considered benign in athletes but are atypical in a sedentary individual. These ECG changes that were once thought to be abnormal are now understood to be training-related changes. To clarify physiologic ECG changes in athletes from pathologic ones, the ESC published a statement with recommendations on ECG interpretation (66). Known as the “Seattle Criteria,” the guidelines for ECG interpretation were updated in 2013 in a collaborative effort between several medical societies, including the ESC, American Medical Society for Sports Medicine, the FIFA Medical Assessment and Research Center, the Pediatric & Congenital Electrophysiology Society (PACES), and leading cardiologists from around the world (67,68,69,70). These are summarized in Table 10.1.
Further, since most normative data for ECGs are based on Caucasian males, they may not extrapolate well to other populations. For instance, female adolescent athletes often have T-wave inversions in the right precordial leads, which may be mistaken for ARVC (71). Research has also shown that ethnicity impacts physiologic responses to exercise and, as such, may manifest with ECG patterns that are classified as abnormal. This is especially true of athletes of African and Afro-Caribbean descent. In a study by Magalski et al. (72) evaluating ECG patterns in 1,959 elite male football players in the United States, the authors found
marked repolarization abnormalities, often limited to the right precordial leads (V1 to V4), in 30% of African American athletes compared with 13% of Caucasian athletes. Another study from the United Kingdom suggested that certain electrocardiographic abnormalities found in black athletes may be variants of normal and not evidence of pathology. This study demonstrated that black athletes with ECG abnormalities limited to the right precordial leads did not have evidence of cardiomyopathy by echocardiography and, when followed long-term, these athletes had no increased cardiovascular morbidity or mortality (73). Indeed, a recent study from London, England that refined ECG criteria even further, continued to find that black athletes were at least 2.2 times more likely to have an abnormal ECG when compared to white athletes (74).
marked repolarization abnormalities, often limited to the right precordial leads (V1 to V4), in 30% of African American athletes compared with 13% of Caucasian athletes. Another study from the United Kingdom suggested that certain electrocardiographic abnormalities found in black athletes may be variants of normal and not evidence of pathology. This study demonstrated that black athletes with ECG abnormalities limited to the right precordial leads did not have evidence of cardiomyopathy by echocardiography and, when followed long-term, these athletes had no increased cardiovascular morbidity or mortality (73). Indeed, a recent study from London, England that refined ECG criteria even further, continued to find that black athletes were at least 2.2 times more likely to have an abnormal ECG when compared to white athletes (74).
TABLE 10.1 Classification of Abnormalities of the Athlete’s ECG | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Economic Consequence of Preparticipation Screening
When examining the cost–benefit ratio of preparticipation screening, one has to examine the cost of the ECG added to the cost of history and physical examination alone. There is high variability in reports of cost-effectiveness estimates based on different statistics used for rates of SCD, false-positive rates of ECG, and costs of ECG and additional screening (75). For example, since there are >10 million athletes in the United States, if an ECG costs $50, it would cost $500 million for electrocardiographic screening of all athletes. Based on the Italian experience, we would estimate that 890,000 ECGs would be positive in the United States. This will result in ordering an echocardiogram at an approximate cost of $1,500 per test. Thus, the total cost of an Italian/European-based screening program in the United States would be $1.84 billion. This is coupled with the above data and probably underestimates EKG and echo costs.
Fuller et al. (76) assessed the cost-effectiveness of using history and physical examination alone with the addition of a 12-lead ECG and found that adding an ECG was more cost-effective, costing $44,000 per life-year saved if ECG screening was used on high school athletes in the United States compared with $84,000 per life-year saved by history and examination alone. Recently, Wheeler et al. (77) evaluated the cost-effectiveness of preparticipation screening of US athletes from ages 14 to 22 using cardiac-focused history and physical examination alone or with the addition of ECG. The authors found that the addition of 12-lead ECG saves 2.1 life-years per 1,000 athletes screened ($89 per athlete) with a cost-effectiveness ratio of $42,900 per life-year saved compared with history and physical examination alone.
Startup costs of a preparticipation screening program in countries such as the United States, where there is not an established program in place, would be significant. There are not only costs in developing the necessary infrastructure for both the evaluation as well as the treatment of athletes but also the costs for physicians who would need to undergo more formalized training. There are also the costs and resources needed for those who have ECG abnormalities, since they almost always necessitate further evaluation, including visits with a cardiovascular specialist and noninvasive imaging, such as echocardiography. In those for whom the ECG is a false positive, the costs of additional testing are not only financial, but physical and psychological too. The athlete may experience anxiety awaiting further evaluation, will likely be
restricted from exercise, and may be disqualified from the sport. This highlights the need for a program in which quick evaluation and results are provided to those who have abnormalities found at the initial screening process (39,78).
restricted from exercise, and may be disqualified from the sport. This highlights the need for a program in which quick evaluation and results are provided to those who have abnormalities found at the initial screening process (39,78).
Specific Congenital Cardiac Lesions
Shunt Lesions
Atrial Septal Defect, Ventricular Septal Defect, Atrioventricular Septal Defect, and Patent Ductus Arteriosus
When exercise capacity is measured by formal exercise testing in patients with either small or repaired shunt lesions, many patients are found to have a low aerobic capacity (79,80). While residual cardiac disease or, more rarely, a persistent degree of pulmonary hypertension (PHT) with exercise may be the cause of this finding in a few cases (80), the cause in the majority of patients with these shunt lesions is a sedentary lifestyle with physical inactivity. Regular exercise participation, exercise training, and in many cases competitive sports participation may be beneficial for the majority of these patients.
Atrial Septal Defect
In patients with atrial septal defect (ASD), blood flows left to right across the atrial defect during diastole as a consequence of the greater right ventricular compliance. The total amount of blood across the shunt is negligible in small defects, and patients should have a normal exercise capacity. However, in larger defects the greater shunt size leads to right ventricular volume overload that could potentially cause PHT during exercise (80). In addition, this shunt flow may limit preload to the left ventricle at higher heart rates. If this occurs, this may lead to a mildly reduced exercise capacity. After ASD closure, nearly all patients resume full exercise capacity. In the current surgical and interventional era, repair outside of early childhood is rare. Recent studies of children who underwent either surgical or device closure in early childhood demonstrate normal or near normal exercise response (81). Residual PHT or atrial arrhythmias are very rare during childhood or at any age following early childhood repair. The guidelines from the 36th Bethesda Conference do not limit competitive activity in children who have their defects closed. The only patients in whom exercise should be restricted are those with a large ASD and mild pulmonary artery hypertension. Low-intensity competitive sports (IA) are recommended until the defect is closed (82). Exercise studies on middle age and older adults with moderate or larger ASDs have demonstrated significant improvement in exercise capacity following closure of the defects (83). In Europe, the guidelines from the task force of the ESC on sports for children with CHD and a left-to-right shunt (ASD) are for no limitations on physical exercise or sports activities (84). This also holds true for leisure sports and other physical activities.
After interventional device closure of the ASD, the patient may resume light sport activities approximately 1 to 2 weeks after intervention (i.e., when the puncture site at the groin has healed completely). To minimize risk of dislodgement, contact sports should be avoided for 3 to 6 months at which time the device should be completely covered by the endocardium. After surgical patch closure, contact sports should be avoided for 3 to 6 months.
Ventricular Septal Defect
In those patients with highly restrictive ventricular septal defects (VSDs) with a pulmonary-to-systemic flow ratio (Qp/Qs) of <1.5/1, there will only be a small shunt from the left-to-right ventricle (RV). During exercise, the shunt will remain relatively small. In those with moderate-size defects (Qp/Qs 1.5 to 2), there is usually low pulmonary artery pressure and resistance and only mild left ventricular volume overload. In these patients, dynamic exercise is usually well tolerated. Because isometric exercise increases systemic afterload much more than pulmonary afterload, this form of exercise can result in an increase in both pulmonary flow and Qp/Qs, making isometric exercise somewhat less well tolerated. Those patients with large VSD (Qp/Qs >2) with normal pulmonary pressures and resistances have similar exercise hemodynamics compared to those with moderate-size defects. However, if there is PHT, neither dynamic nor isometric exercise is well tolerated. In the presence of significantly elevated PVR, the pulmonary vascular bed is unable to handle the increased blood flow associated with the exercise and right-to-left shunting may occur.
In two studies of children during submaximal exercise testing, reduced values for ventilatory anaerobic threshold were found in about half of 43 patients with native VSD (mean 86 ± 12% of normal) and with surgically closed VSD (mean 86 ± 12% of normal) (69,70). The only variable that correlated with a lower level of exercise performance was greater physical inactivity during daily life. A recent study comparing well-repaired patients with unoperated hemodynamically insignificant VSDs and healthy control subjects showed no difference in aerobic performance between the three groups although the operative group had mild chronotropic impairment (85). Children with a VSD (repaired or unrepaired) should be encouraged to be physically active and to adopt a healthy lifestyle. If low levels of exercise performance are found, increasing physical activity should be encouraged. Previous studies in small groups of children with congenital heart defects, including those with VSD, have shown improvement in maximal work rate on the bicycle ergometer, without a change in maximal VO2 after a 6-week home-based conditioning program (86).
Atrioventricular Septal Defects
Patients with an atrioventricular septal defect (AVSD) who do not have Down syndrome should follow the same recommendations as for VSD. The only exception is those individuals who have significant mitral regurgitation. Those with moderate to severe mitral regurgitation should be restricted from exercise if they have either severe volume overload of the left atrium and/or left ventricle or evidence of PHT. For those patients with Down syndrome, discussion with the primary physician and cardiologist should occur before undertaking any exercise program. While physical activity is important and encouraged in this population, they may need to be restricted from contact sports and other activities that may jar the neck due to the high rate of atlantoaxial instability in people with Down syndrome (87).
Patent Ductus Arteriosus
For patients with a patent ductus arteriosus (PDA), the hemodynamics are similar to those found in patients with VSD. When in isolation, PDA closure is nearly always performed percutaneously. The same recommendations for activity apply as after percutaneous ASD closure.
Evaluation Prior to Exercise and Sports Participation
Most patients with well-repaired or hemodynamically insignificant residual shunt defects need little evaluation beyond routine outpatient care prior to participating in either recreational or competitive athletics. This will generally include a physical examination, ECG, chest x-ray, and echocardiogram. In cases where there is additional concern of significant residual abnormalities or pulmonary disease, additional testing including exercise testing may be needed (82).
Leisure Activities and Activities of Daily Living
In patients with well-repaired or hemodynamically insignificant ASD, VSD, PDA, and AVSD, no limitation exists for leisure activities or activities of daily living. These children can and should
participate normally in physical exercise without restrictions. It is recommended that they perform 60 minutes of moderate to vigorous physical activity 5 days a week or more (24) (Table 10.2).
participate normally in physical exercise without restrictions. It is recommended that they perform 60 minutes of moderate to vigorous physical activity 5 days a week or more (24) (Table 10.2).
TABLE 10.2 Recommendations Following the Frequency, Intensity, Time, and Type (F.I.T.T.) Principle for Recreational Activities and Exercise Training in Children and Adolescents with ASD/VSD/AVSD/PDA and No Hemodynamically Significant Residual Disease | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Competitive Sports
According to the Bethesda guidelines, since most children have ASDs closed before they are active in competitive sports, exercise usually is not restricted. The only patients in whom exercise should be restricted are those with a large ASD (unrepaired) and mild pulmonary artery hypertension. Low-intensity competitive sports (IA) are recommended until the defect is closed (Fig. 10.1). For those athletes with surgical or device closure of an ASD, if there is no evidence of PHT or ventricular or atrial ectopy, they can participate in all sports 3 to 6 months after the operation or device closure (82). For those with VSDs, athletes with small to moderate defects and normal pulmonary artery pressure can participate in all sports while those with large defects and normal pulmonary artery pressure can participate after VSD closure. Those with large unrepaired defects and elevated pulmonary vascular resistance cannot participate in competitive sports (82). For those who want to participate in competitive athletics after surgical or device closure of a VSD, if there is no evidence of PHT or ventricular or atrial ectopy, the patient can participate in all sports 3 to 6 months after successful intervention (82). Limitations for competitive sports are only in patients with pulmonary arterial hypertension (PAH) (84). Information on exercise recommendations for the patients with PHT can be found in the section on PHT later in this chapter.
Left-Sided Obstructive Lesions
Aortic Stenosis
Congenital aortic stenosis occurs in three major subtypes: subvalvar, valvar, and supravalvar. Subvalvar disease is a result of a subaortic muscular ridge, a fibromuscular ridge and/or tunnel, or a discreet subaortic membrane. Subaortic obstruction is also associated with a distorted aortic valve leaflet resulting in regurgitation. Congenitally stenotic aortic valves can be isolated, as is seen in the unicommissural or bicommissural lesions, or they can be found in association with posterior malalignment type VSD, abnormalities of the mitral valve, hypoplasia of the aortic arch, and aortic coarctation. Supravalvar aortic stenosis at the sinotubular junction is typically seen in patients with Williams syndrome, in familial supraaortic stenosis and rarely with familial dyslipidemias (88,89), or as spontaneous mutations in otherwise normal individuals. Except in severe cases or in the presence of other significant defects, exercise performance is usually normal or near normal.
Evaluation Prior to Exercise and Sports Participation
It is important to distinguish symptomatic from asymptomatic patients who have aortic stenosis. A previous history of exercise-induced syncope or lightheadedness, dyspnea with exercise without an ostensible pulmonary etiology, or angina may indicate that these patients are at higher risk of SCD compared to asymptomatic patients. These patients therefore should be evaluated for possible surgery or catheter-based interventions. Guidelines grading the degree of stenosis have been previously reported and have been used to make recommendations regarding sports participation in competitive athletes. However, these guidelines are admittedly conservative and based upon scant literature (82). Most of the previously reported patients with aortic stenosis who died suddenly had a high incidence of ECG abnormalities. LVH and/or strain should be assessed when making recommendations for competitive sports. Graded exercise testing may be helpful in unmasking important findings, such as blunted blood pressure response or ventricular ectopy, in asymptomatic patients. Metabolic exercise testing is useful in identifying high-risk older adults but is almost never abnormal in the pediatric and adolescent population and probably adds little to risk stratification (90).
Leisure Activities and Activities of Daily Living
As stated above, most patients with aortic stenosis are asymptomatic and have normal exercise tolerance. Patients with mild disease need no restrictions and should follow the recommendations in Table 10.2. Patients with moderate stenosis should follow the recommendations for bicuspid aortic valve syndrome (Table 10.3). It is unknown if regular physical training slows the progression of stenosis or insufficiency in this disorder. It is believed that repetitive, maximally strenuous isometric exercise may hasten valve deterioration; therefore, these activities should be minimized or avoided completely. Physical training, however, is helpful in hastening recovery in patients with CHD who have had surgical intervention (91,92,93).
Competitive Sports
Patients with severe valvar aortic stenosis are at risk for SCD during exercise (30,94). However, the true risk is unknown. In adults, syncope, dizziness, angina pectoris, or dyspnea with exercise are associated with SCD. Recent data suggest that the risk of SCD during exercise in patients who had balloon valvuloplasty as infants may not be as high as previously believed (95). Until more data are available, the guidelines from the 36th Bethesda Conference are probably reasonable. Athletes with mild aortic stenosis, a normal resting ECG, and no history of exercise-related symptoms can participate in all forms of competitive sports. Patients with mild stenosis should be reevaluated periodically to continue with
competitive sports. Athletes with moderate aortic stenosis who have mild or no LVH, normal response to treadmill exercise testing, and no exercise-induced symptoms can participate in low static/low-to-moderate dynamic and moderate static/low-to-moderate dynamic exercise (classes IA, IB, and IIA) (Fig. 10.1). Individualized exercise prescriptions in borderline cases are reasonable in light of the finding of the lower risk of sudden death than was previously believed (95).
competitive sports. Athletes with moderate aortic stenosis who have mild or no LVH, normal response to treadmill exercise testing, and no exercise-induced symptoms can participate in low static/low-to-moderate dynamic and moderate static/low-to-moderate dynamic exercise (classes IA, IB, and IIA) (Fig. 10.1). Individualized exercise prescriptions in borderline cases are reasonable in light of the finding of the lower risk of sudden death than was previously believed (95).
TABLE 10.3 Recommendations Following the F.I.T.T. Principle for Recreational Activities and Exercise Training in Children and Adolescents with Bicuspid Aortic Valve Syndromea | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree