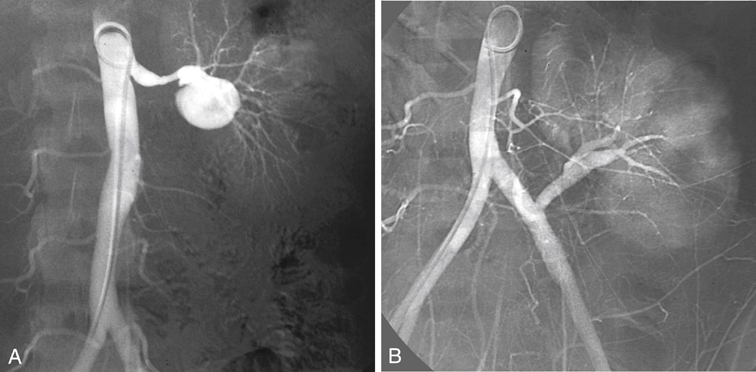
Fibrodysplastic disease of the renal arteries is bilateral in more than half of the patients, and involvement of the primary branches of the renal artery has been reported in as many as 40%. In most patients the fibrodysplastic lesions are located in the distal renal artery, but many lesions affect multiple smaller renal arteries or extend into the primary and secondary branches of the renal artery. In these latter patients, percutaneous angioplasty is not without complications, and dissection, occlusion, renal infarction, perforation, and contrast-induced kidney failure have been reported (Figure 1). Open reconstructions are favored in many of these patients, yet prospective data comparing the results of transluminal angioplasty and open surgical repair are available for treatment of atherosclerotic lesions but not for fibrodysplasia. The advantages of ex vivo reconstruction are optimal exposure, a bloodless surgical field, protection of the kidney from prolonged warm ischemia, no time limit for microvascular repair, and contraction of the kidney resulting from preservation, which allows an easier dissection. Finally, the complete reconstruction can be tested for both patency and leakage before implantation. Compared with in vivo repair, ex vivo repair is associated with longer operation times, more blood loss, and more wound hematomas. Although it has been suggested that stenosis at the renal bifurcation can be repaired in situ, results of extracorporeal repair appear to be superior. Standard indications for performing ex vivo arterial repair exist (Box 1).
Ex Vivo Arterial Repair for Renovascular Hypertension Secondary to Fibrodysplasia
Therapy
Thoracic Key
Fastest Thoracic Insight Engine



