Treatment of coronary artery disease has significantly changed over the past decade including an introduction of drug-eluting stents and a more stringent adherence to evidence-based medications. However, the impact of these advanced treatment methods on the practice patterns and long-term outcomes in patients undergoing coronary revascularization in the real world has not been yet fully evaluated. The present study population consisted of the 2 groups of patients who underwent their first coronary revascularization in the Coronary REvascularization Demonstrating Outcome Study in Kyoto Registry Cohort-1 (bare-metal stent era: January 2000 to December 2002, n = 8,986) and Cohort-2 (drug-eluting stent era: January 2005 to December 2007, n = 10,339). Compared with Cohort-1, the proportion of patients treated with percutaneous coronary intervention significantly increased in Cohort-2 (73% vs 81%, p <0.001), particularly for 3-vessel disease (50% vs 61%, p <0.001) and left main disease (18% vs 36%, p <0.001). Evidence-based medications were more frequently used in Cohort-2. The cumulative 2-year incidence of and the adjusted risk for all-cause death were not significantly different between Cohort-1 and Cohort-2 (6.2% vs 6.4%, p = 0.69, and hazard ratio [HR] 0.91, 95% confidence interval [CI] 0.81 to 1.03, p = 0.15). Adjusted risks for both myocardial infarction and repeated coronary revascularization were significantly reduced in Cohort-2 compared with Cohort-1 (HR 0.80, 95% CI 0.67 to 0.96, p = 0.02, and HR 0.73, 95% CI 0.69 to 0.77, p <0.001, respectively). In conclusion, despite changes in treatment methods over time, the long-term mortality of patients undergoing coronary revascularization in the real-world clinical practice has not been changed, although there was a significant reduction of myocardial infarction and repeated coronary revascularization.
Over the last 10 years, coronary revascularization procedures and medical therapies in patients with coronary artery disease (CAD) have changed dramatically. One of the most notable changes was the introduction of drug-eluting stents (DES) in percutaneous coronary intervention (PCI), although the use of arterial graft and off-pump surgery has become more common in coronary artery bypass grafting (CABG), and evidence-based medicines such as statins, angiotensin-converting enzyme inhibitor, and antiplatelet drugs are also more commonly used for the primary and secondary prevention of cardiovascular events. However, the impact of DES introduction on the practice patterns and long-term outcomes in patients with CAD undergoing coronary revascularization in the real world has not yet been fully evaluated. To address this issue, we conducted a historical comparison between the 2 large-scale cohorts of patients who underwent coronary revascularization before the introduction of DES (bare-metal stent [BMS] era) and after the introduction of DES (DES era).
Methods
The Coronary REvascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) Registry Cohort-1 was a physician-initiated, noncompany sponsored, multicenter registry that enrolled consecutive patients undergoing their first coronary revascularization in 30 centers in Japan from January 2000 to December 2002, when only BMS was available (BMS era). The CREDO-Kyoto Registry Cohort-2 was the similarly designed multicenter registry that enrolled consecutive patients undergoing first coronary revascularization in 26 centers in Japan from January 2005 to December 2007 after the introduction of DES (DES era). The patients with acute myocardial infarction within a week before the index procedure were not enrolled in Cohort-1 but were enrolled in Cohort-2.
The relevant ethics committees in all participating centers ( Supplementary Appendix A ) approved the research protocol. Because of retrospective enrollment, written informed consents from the patients were waived; however, we excluded those patients who refused participation in the study when contacted for follow-up. This strategy is concordant with the guidelines for epidemiologic studies issued by the Ministry of Health, Labor and Welfare of Japan.
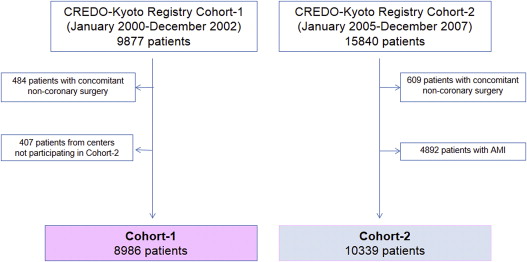
To make Cohort-1 and Cohort-2 comparable, we excluded 407 patients in Cohort-1 who were enrolled from 4 PCI centers and 1 cardiovascular surgery division not participating in Cohort-2 and 4,892 patients with acute myocardial infarction in Cohort-2. After excluding patients with concomitant noncoronary surgery and patients with refusal for study participation, the study population for the present analysis consisted of 19,325 patients with CAD who underwent their first isolated coronary revascularization (Cohort-1: n = 8,986 and Cohort-2: n = 10,339; Figure 1 ).
Demographic, angiographic, and procedural data in both cohorts were collected from hospital charts according to prespecified definitions by the experienced research coordinators in the independent research organization (Research Institute for Production Development, Kyoto, Japan; Supplementary Appendix B ). Definitions for clinical characteristics are described in Supplementary Methods .
The primary outcome measure was all-cause death. Other prespecified end points included cardiac death, myocardial infarction (MI), stroke, and repeated coronary revascularization. Death was regarded as cardiac in origin unless obvious noncardiac causes could be identified. Any death during the index hospitalization for coronary revascularization was regarded as cardiac death. MI was defined according to the definition in the Arterial Revascularization Therapy Study. Stroke was defined as ischemic or hemorrhagic stroke either occurring during the index hospitalization or requiring hospitalization with symptoms lasting >24 hours. Repeated coronary revascularization was defined as either PCI or CABG for any reason. Scheduled staged coronary revascularization procedures performed within 3 months of the initial procedure were not regarded as follow-up events but were included in the index procedure. All the definitions for clinical characteristics and end points were identical between Cohort-1 and Cohort-2.
Collection of follow-up information was mainly conducted through the review of hospital charts by the clinical research coordinators in the independent research organization. Additional follow-up information was collected through contact with patients, relatives, and/or referring physicians by sending mails with questions regarding vital status, subsequent hospitalizations, and status of antiplatelet therapy. Clinical events such as death, MI, and stroke were adjudicated by the clinical event committee ( Supplementary Appendix C ). All follow-up events were censored at 2 years after the index procedure in both Cohort-1 and Cohort-2 to balance the follow-up durations between Cohort-1 and Cohort-2. Complete 1- and 2-year follow-up information was obtained for 97.9% and 95.6% of patients in Cohort-1 and 98.5% and 97.5% of patients in Cohort-2, respectively.
Categorical variables are presented as number and percentage and were compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous variables are expressed as the mean ± SD or the median with interquartile range and were compared using the Student t test or the Wilcoxon rank sum test based on their distributions.
Cumulative incidence was estimated by the Kaplan-Meier method, and differences were assessed with the log-rank test. The effects of Cohort-2 relative to Cohort-1 for individual end points were expressed as hazard ratios (HRs) and their 95% confidence intervals (CIs). We used Cox proportional hazards models to estimate the HR of Cohort-2 compared with Cohort-1 by adjusting for 14 clinically relevant factors (listed in Table 1 ), and we computed the adjusted event curves of the 2 cohorts using the methods described by Ghali et al. Continuous variables were dichotomized by clinically meaningful reference values or median values. Proportional hazard assumptions for potential independent risk-adjusting variables were assessed on the plots of log (time) versus log [−log (survival)] stratified by the variable, and the assumptions were verified to be acceptable for all variables. The Cox proportional hazards models were similarly constructed in the subgroup analyses in the PCI stratum, in the CABG stratum, and in patients with severe CAD defined as 3-vessel disease (3VD) and/or left main coronary artery disease (LMCAD).
| Characteristic | Cohort-1 (n = 8,986) | Cohort-2 (n = 10,339) | p Value |
|---|---|---|---|
| Clinical characteristics | |||
| Age (yrs) | 67.2 ± 10.1 | 68.5 ± 10.1 | <0.001 |
| ≥75 yrs ∗ | 2,202 (25) | 3,152 (30) | <0.001 |
| Men ∗ | 6,351 (71) | 7,470 (72) | 0.02 |
| Body mass index (kg/m 2 ) | 23.7 ± 3.3 | 23.7 ± 3.4 | 0.09 |
| <25.0 kg/m 2 ∗ | 6,102 (69) | 6,997 (68) | 0.04 |
| Hypertension ∗ | 6,211 (69) | 8,722 (84) | <0.001 |
| Diabetes mellitus ∗ | 3,527 (39) | 4,367 (42) | <0.001 |
| On insulin therapy | 745 (8.4) | 1,125 (11) | <0.001 |
| Current smoker ∗ | 2,470 (28) | 2,725 (26) | 0.01 |
| Heart failure ∗ | 1,355 (15) | 1,597 (15) | 0.55 |
| Ejection fraction (%) | 62.3 ± 13.3 | 60.6 ± 13.0 | <0.001 |
| ≤40% | 613 (7.6) | 784 (8.7) | 0.006 |
| Previous MI ∗ | 2,294 (26) | 1,656 (16) | <0.001 |
| Previous stroke ∗ | 1,486 (17) | 1,716 (17) | 0.93 |
| Peripheral vascular disease ∗ | 580 (6.5) | 1,078 (10) | <0.001 |
| eGFR (ml/min/1.73 m 2 ) | 64.2 (52.0–76.5) | 63.0 (49.7–75.2) | <0.001 |
| eGFR <30, without hemodialysis ∗ | 345 (3.9) | 461 (4.5) | 0.053 |
| Hemodialysis ∗ | 362 (4.0) | 524 (5.1) | <0.001 |
| Anemia (hemoglobin <11.0 g/dl) ∗ | 1,201 (14) | 1,411 (14) | 0.95 |
| Malignancy ∗ | 625 (7.0) | 1,043 (10) | <0.001 |
| Procedural characteristics | |||
| Number of coronary arteries narrowed | 0.23 | ||
| 1 | 2,900 (32) | 3,431 (33) | |
| 2 | 2,495 (28) | 2,926 (28) | |
| 3 | 2,691 (30) | 2,978 (29) | |
| ULMCA narrowing | 900 (10) | 1,004 (9.7) | |
| Multivessel coronary narrowing | 5,813 (65) | 6,624 (64) | 0.37 |
| Chronic total occlusion | 2,709 (30) | 2,885 (28) | <0.001 |
| Number of target coronary arteries (median [IQR], mean ± SD) | 1 (1–2), 1.59 ± 0.77 | 1 (1–2), 1.59 ± 0.75 | 0.75 |
| PCI | 6,517 (73) | 8,337 (81) | <0.001 |
| Stent use | 5,346 (82) | 7,871 (94) | <0.001 |
| DES use | — | 5,369 (64) | |
| CABG surgery | 2,469 (27) | 2,002 (19) | <0.001 |
| Off pump | 1,067 (43) | 1,298 (65) | <0.001 |
| Internal thoracic artery use | 2,316 (94) | 1,960 (98) | <0.001 |
| Baseline medications | |||
| Antiplatelet therapy | |||
| Thienopyridine | 5,193 (58) | 8,376 (81) | <0.001 |
| Aspirin | 7,900 (88) | 10,183 (98) | <0.001 |
| Cilostazol | 718 (8.0) | 1,037 (10) | <0.001 |
| Other medications | |||
| Statins | 2,553 (28) | 4,813 (47) | <0.001 |
| β Blockers | 1,486 (17) | 2,621 (25) | <0.001 |
| ACE-I/ARB | 2,969 (33) | 4,868 (47) | <0.001 |
| Nitrates | 5,653 (63) | 3,960 (38) | <0.001 |
| Calcium channel blockers | 5,365 (60) | 5,312 (51) | <0.001 |
| Warfarin | 1,041 (12) | 1,343 (13) | 0.004 |
∗ Potential independent variables selected for Cox proportional hazards models.
All statistical analyses were conducted using JMP 8.0 software (SAS Institute Inc., Cary, North Carolina). All reported p values were 2-tailed, and p values <0.05 were considered statistically significant.
Results
Baseline characteristics were significantly different in several aspects between Cohort-1 and Cohort-2 ( Table 1 ).
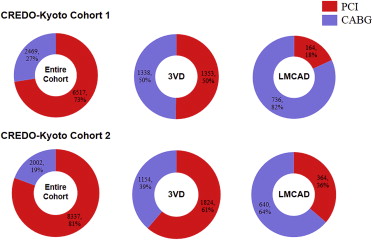
There was no significant difference between Cohort-1 and Cohort-2 with regard to angiographic findings ( Table 1 ). The choice of revascularization procedure, however, significantly changed during the study period ( Figure 2 ). Compared with Cohort-1, the rate of PCI in Cohort-2 was significantly increased (73% vs 81%, p <0.001), particularly for 3VD (50% vs 61%, p <0.001) and LMCAD (18% vs 36%, p <0.001). Furthermore, procedural characteristics also changed significantly. In PCI, the rate of stent use increased in Cohort-2 compared with Cohort-1 (94% vs 82%, p <0.001), and DES was used in 64% of the patients who underwent PCI in Cohort-2. The uses of internal thoracic artery as a bypass graft and off-pump surgery were more common in Cohort-2 compared with Cohort-1 (98% vs 94%, p <0.001, and 65% vs 43%, p <0.001, respectively).
Evidence-based medications such as statins, antiplatelet agents, β blockers, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were more frequently used in Cohort-2 than in Cohort-1 ( Table 1 ).
Baseline characteristics in PCI stratum, CABG stratum, and the subgroup of severe CAD are available in Supplementary Tables 1, 2, and 3 , respectively.
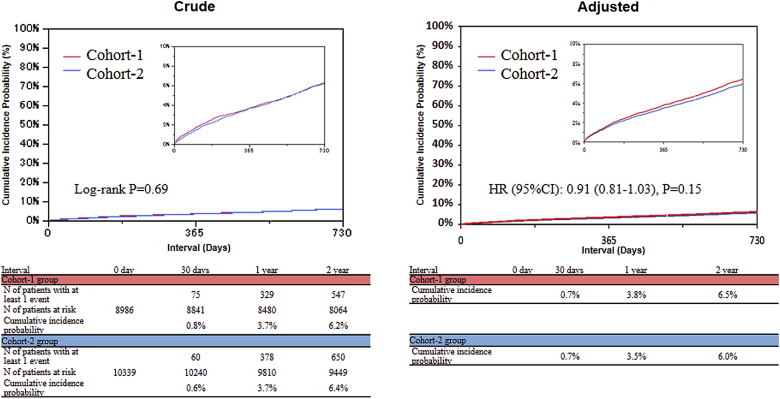
The cumulative 2-year incidence of all-cause death was not significantly different between Cohort-1 and Cohort-2 (6.2% vs 6.4%, p = 0.69, respectively; Figure 3 ). After adjusting for potential confounders, there remained no significant difference in the risk for all-cause death between the 2 cohorts (HR 0.91, 95% CI 0.81 to 1.03, p = 0.15; Figure 3 and Table 2 ). The cumulative incidence of cardiac death was also not significantly different between the 2 cohorts (3.3% vs 3.1%, p = 0.30). After adjusting for confounders, however, the risk for cardiac death was significantly reduced in Cohort-2 compared with Cohort-1 (HR 0.84, 95% CI 0.71 to 0.997, p = 0.047; Table 2 ). The adjusted risk for MI was significantly reduced in Cohort-2 compared with Cohort-1 (HR 0.80, 95% CI 0.67 to 0.96, p = 0.02), although the cumulative incidence of MI was not significantly different between the 2 cohorts (2.9% vs 2.5%, p = 0.11; Figure 4 and Table 2 ). The cumulative incidence of repeated coronary revascularization significantly decreased from Cohort-1 to Cohort-2 (29.7% vs 24.2%, p <0.001; Figure 5 ). The adjusted risk for repeated coronary revascularization was significantly reduced in Cohort-2 compared with Cohort-1 (HR 0.73, 95% CI 0.69 to 0.77, p <0.001; Figure 5 and Table 2 ). The adjusted risk for stroke was not significantly different between the 2 cohorts (HR 0.97, 95% CI 0.82 to 1.14, p = 0.71).