2 Evidence-Based Interventional Practice
 Introduction
Introduction
Changing Paradigms of Coronary Revascularization
When the era of interventional cardiology began, with the pioneering work of Andreas Grüntzig on plain balloon angioplasty, percutaneous coronary intervention, PCI ( for list of abbreviations and acronyms see Table 2-1), was a treatment option only for isolated proximal coronary lesions not involving the ostium or the left main stem. In the late 1980s, coronary stents were developed with the goal of reducing the risk of restenosis and achieving a more predictable acute result of angioplasty, thus avoiding the dreaded abrupt closure due to dissection. As shown subsequently, stents were successful in achieving this goal. Nevertheless, they created a new problem: subacute stent thrombosis. After intense research on peri- and postinterventional antithrombotic treatment, the concept of dual or triple antiplatelet therapy emerged, which significantly reduced the incidence of this complication. The use of coronary stents in conjunction with optimized antithrombotic treatment extended the spectrum of coronary lesions for which PCI was considered a reasonable treatment option and thereby led to a substantial expansion of interventional techniques. Because of the large number of patients who were now being treated with coronary stents, restenosis due to neointima formation became a serious problem. Although various studies demonstrated that stents, compared with plain balloon angioplasty, reduced the need for reintervention, restenosis rates continued to be relevant, ranging from just above 10% in the simplest lesions to more than 50% with diffuse disease in patients with diabetes.
TABLE 2-1 List of Abbreviations Acronyms
Thus it is not surprising that the community of interventional cardiologists celebrated the advent of the new drug-eluting stents as a major breakthrough, given that the initial studies suggested zero restenosis rates. In the meantime, it has become clear that drug-eluting stents compared with bare metal stents reduce the need for target-vessel reintervention by around 80%, thus largely reducing but not eliminating the problem of restenosis. Subsequently, drug-eluting stents led to another massive expansion of the proportion of patients treated with PCI. With the widespread use of these stents for PCI, reports appeared pointing to a new problem that had not been seen with bare metal stents: that is, late stent thrombosis. Yet a thorough reevaluation of the data from randomized studies—with uniform application of definitions for definite, probable, and possible stent thrombosis—failed to confirm these alarming initial reports.1 Nevertheless, there may be a slight increase in the risk of very late (>1 year) stent thrombosis after the placement of drug-eluting stents as compared with bare metal stents.2 It is, however, reassuring that the risk of serious late complications such as death and myocardial infarction (MI) has never been shown to be higher with drug-eluting stents than with bare metal stents.2 In some high-risk instances, drug-eluting stents may even improve survival.3 Despite the remaining problems of PCI, its use has increased exponentially over the past decades. Initially, this increase has come at the expense of lone medical therapy. More recently, however, with the advent of drug-eluting stents, there has been a shift of patients with multivessel disease and other complex coronary anatomies from CABG to PCI. This shift has been facilitated by both physician and patient preference for the supposedly easier approach to coronary revascularization, given the idea that the problem of restenosis has been largely solved. There is, however, reasonable concern that this shift has led to the overuse of PCI and that, in some patients, it may not yield the same outcome as CABG, which for a number of indications is an established treatment option with a well-documented survival benefit compared with medical therapy.
 Prognostic Indications for Coronary Revascularization
Prognostic Indications for Coronary Revascularization
Clinical Presentation
Myocardial Infarction with ST-Segment Elevation
In acute MI, as shown by a metanalysis of the randomized trials in this setting, fibrinolysis reduces mortality by 18% as compared with conservative treatment.4 On top of this benefit, coronary reperfusion by primary PCI reduces in-hospital mortality by an additional 37%.5 In addition to its effect on survival, PCI compared with fibrinolysis reduces the risk of reinfarction and stroke, particularly that of hemorrhagic stroke,6 and the initial benefit is maintained during long-term follow-up.6 The largest survival benefit by PCI is obtained when the delay conferred by PCI compared with fibrinolysis is shorter than 35 minutes.5 Nevertheless, even with delays by PCI compared with fibrinolysis ranging between 35 and 120 minutes, there is a significant survival benefit by PCI ranging around 24% on average.5 Prespecified subgroup analyses of the FINESSE study suggest that even with delays as long as 2.55 to 4 hours, direct PCI is the preferred strategy in terms of safety and efficacy.7 Although fibrinolysis is more effective within the first 1 to 3 hours after the onset of pain than after larger delays, the benefit from PCI as compared with fibrinolysis is largely independent of the time from onset of pain to intervention.5 CABG in the setting of MI, although it can be performed, delays reperfusion compared with PCI and is associated with a high perioperative risk. Hence CABG has only a niche indication in this setting. In summary, acute MI is an accepted and well-documented prognostic indication for PCI.
Acute Coronary Syndromes without ST-Segment Elevation
There has been a long-standing debate about two competing treatment strategies for acute coronary syndromes without ST-segment elevation.8 The conservative strategy reserves coronary angiography and revascularization to those patients who continue to have a spontaneous or inducible myocardial ischemia despite maximal medical therapy. On the other hand, the invasive strategy suggests coronary angiography and revascularization irrespective of the primary success of medical treatment. Various studies have addressed this issue. A metanalysis published in 2005 concluded that the invasive strategy, while increasing the risk of in-hospital death and MI (early hazard), significantly reduced death and MI during the entire follow-up—ranging from 6 months to 2 years in various studies—by 18% (95% confidence interval, 2% to 42%).9 Supporting this analysis, the 5-year follow-up of RITA-3 revealed that, as compared with the conservative strategy, the benefit of the invasive strategy with respect to death and MI continued to increase with time.10 At 5 years after intervention, the incidence of death and MI was 20.0% in the conservative arm but 16.6% in the interventional arm (P = 0.04). Moreover, there was an increased survival benefit of the invasive strategy as compared with the conservative strategy during the 5-year follow-up (88% vs. 85%), which almost reached statistical significance (P = 0.054). The 5-year follow-up of FRISC-II also demonstrated a significant reduction in the long-term incidence of death and MI by the invasive strategy as compared with the conservative strategy (5-year incidence 19.9% vs. 24.5%, P = 0.009).11 The benefit from the invasive strategy compared with the conservative one is not uniform across the spectrum of acute coronary syndromes. The pivotal studies—FRISC-II, TACTICS-TIMI 18, and RITA-312–14—consistently show that the benefit from the invasive strategy is linked to various markers of risk, whereas patients without these risk markers may be treated according to the same principles as patients with stable angina. The risk factors that could be established in previous studies include elevated myocardial marker proteins, dynamic ST-segment changes, ongoing myocardial ischemia, hemodynamic instability, and diabetes mellitus.15 This concept of a routine invasive strategy has recently been challenged by the ICTUS trial.16 It accepts the need for coronary revascularization in the majority of patients but challenges troponin levels as the sole criterion for revascularization. It randomized 1,200 patients to a routine invasive versus a selective invasive strategy. To be included, patients had to have unstable angina with elevated cardiac troponin levels. During a 1-year follow-up, 54% of the patients in the selectively invasive arm and 76% of those in the routine invasive arm underwent coronary revascularization. It is noteworthy that the rate of coronary revascularization in the conservative arm of ICTUS was as high as that in the invasive arm of RITA-3. During 1-year follow-up, the primary endpoint of ICTUS—which was death or MI and hospital readmission for unplanned coronary revascularization—was not significantly different between the two treatment arms. Secondary analyses, however, revealed a significant increase in MIs in the invasive arm (15% vs. 10%), which could be attributed to an early hazard of the intervention. The ICTUS trial is consistent with other previous trials suggesting that there is a need for revascularization in the majority of patients presenting with high-risk acute coronary syndromes. As a new aspect, ICTUS suggests that even among patients with positive troponins, there is a low-risk subset in whom the long-term benefit from revascularization cannot compensate for the incidence of peri-interventional complications. In this respect, ICTUS challenges the elevation of myocardial marker proteins as the only criterion for recommending revascularization. As published recently, the increased incidence of peri-interventional MI with routine invasive as compared with selective invasive strategy had no significant impact on 5-year survival or survival free of MI.17 Hence there does not appear to be a long-term down side to the routine invasive strategy. Therefore taking advantage of the other benefits of the routine invasive strategy—such as a shorter hospital stay and lower need for unplanned revascularization—may be justified.
Stable Angina—Severe Angina or Large Ischemic Area
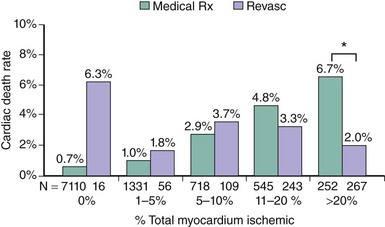
Among patients with chronic stable angina, those with severe angina, large or multiple perfusion defects on functional testing, or a low threshold for the induction of ischemia (Table 2-2) have a poor prognosis with an annual mortality risk >3%. If these high-risk features are associated with double- or triple-vessel disease, patients benefit from revascularization irrespective of left ventricular function. In an analysis of 5,303 patients in the CASS registry, surgical benefit was greatest in patients who exhibited at least 1 mm of ST-segment depression and could exercise only into stage 1 or less. In the surgical group with triple-vessel disease and severe exercise-induced ischemia, 7-year survival was 81%, whereas it was 58% in the corresponding medical group.18 Likewise, in another registry including 2,023 patients with severe angina and two-vessel disease, 6-year survival was 76% in patients treated medically and 89% in patients treated surgically (P < 0.001).19 Cox multivariate analyses showed that surgical treatment was a beneficial independent predictor of survival for patients with two-vessel coronary disease and Canadian Cardiovascular Society class 3 or 4 angina. ACIP is a more recent trial that was designed to compare the efficacy of medical therapy versus revascularization.20 In ACIP, 558 patients with angiographically documented coronary artery disease, mostly multivessel disease, and stable coronary artery disease were randomly assigned to medical therapy, adjusted either to suppress angina or both angina and evidence of ischemia during ambulatory ECG monitoring or revascularization with either PCI or CABG. Revascularization was significantly more effective in relieving ischemia than either of the medical strategies. During 1-year follow-up, the ACIP trial appeared to show better outcome in patients treated with revascularization. Mortality was 4.4% and 1.6% in the two conservative groups, whereas none of the patients in the revascularization group had died during the 1-year follow-up period. The apparent benefit of revascularization was largely confined to patients with double- or triple-vessel disease. A registry of 10,627 consecutive patients who underwent exercise or adenosine myocardial perfusion single photon emission computed tomography (SPECT) demonstrated that patients with large ischemic areas on functional testing benefit from revascularization. The patients included in this retrospective analysis had no prior MI or revascularization and were followed for a mean of 1.9 years. The treatment received within 60 days of stress testing was revascularization by either CAGB or PCI in 671 patients and medical therapy in 9,956 patients. To adjust for nonrandomization of treatment, a propensity score was developed. On the basis of the Cox proportional hazards model predicting cardiac death, patients undergoing medical therapy demonstrated a survival advantage over patients undergoing revascularization in the setting of no or mild ischemia, whereas those undergoing revascularization had an increasing survival benefit over patients undergoing medical therapy when moderate to severe ischemia was present (Figure 2-1).21 Consistent results were obtained in a nuclear substudy on 314 patients of the COURAGE study.22 In this substudy, the extent of residual posttreatment ischemia—assessed as percentage of the left ventricle by myocardial perfusion SPECT—was a predictor of outcome: rates of death or MI ranged from 0% to 39% for patients with no residual ischemia to ≥10% residual ischemia despite treatment, (P = 0.002 [risk-adjusted P = 0.09]) (Figure 2-2). With respect to treatment, a ≥5% reduction in ischemic myocardium lowered the risk of death or MI (P = 0.037 [risk-adjusted P = 0.26]), particularly if baseline ischemia was ≥10% (P = 0.001 [risk-adjusted P = 0.08]). PCI on top of optimal medical therapy increased the likelihood of achieving this goal. The findings of this substudy suggest that revascularization is indicated if, in addition to optimal medical therapy, it affords at least a 5% reduction in myocardial ischemia.
TABLE 2-2 Poor Prognosis in Stable Angina (Average Annual Mortality Risk > 3%)
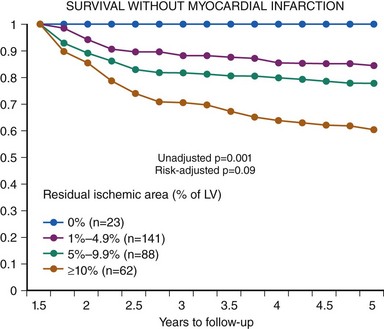
Figure 2-2 Survival without myocardial infarction depending on residual ischemic area.
(Reproduced with permission from Shaw LJ, Berman DS, Maron DJ, et al: Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 117(10):1283–1291,2008.)
Coronary Anatomy
Until now our understanding of the anatomical conditions that constitute a survival benefit from coronary revascularization as compared with lone medical therapy is largely based on milestone studies performed during the 1970s. Soon after CABG was introduced in 1969, three randomized trials compared surgical revascularization with lone medical therapy: the VA Study, ECSS, and CASS. Although these studies are outdated in many respects, including a low use of arterial conduits and limited means of pharmacological risk factor modification and platelet inhibition, it is unlikely that they will ever be replicated. In concert with analyses of large registry databases, the early studies established the conditions in which CABG improves survival as compared with medical therapy (Table 2-3). A metanalysis of all published randomized trials of CABG versus lone medical treatment for coronary artery disease identified left main disease (diameter of stenosis ≥ 50%), multivessel disease, and involvement of the proximal left anterior descending coronary artery (LAD) as significant predictors of a survival benefit from CABG.23 In the cumulative experience of seven studies, the VA study being the first, surgical revascularization for left main disease was associated with a 65% relative reduction in mortality as compared with lone medical therapy.23 Notably, in left main disease there was a survival benefit of surgery irrespective of the presence or absence of spontaneous or inducible symptoms or signs of ischemia or reduced left ventricular function. The same is also true for triple- or double-vessel disease involving the proximal LAD.24
TABLE 2-3 Conditions in Which CABG Improves Survival as Compared with Medical Therapy
Triple- or double-vessel disease in the presence of severe angina or large areas of ischemia on functional testing |
In all other conditions, the indication for surgical coronary revascularization depends on a combination of anatomical and clinical criteria. If triple-vessel disease is associated with impaired LV function (LV ejection fraction < 50%), surgical revascularization improves survival irrespective of LAD involvement.25,26 In the presence of severe angina or large areas of ischemia on functional testing, surgical revascularization of triple- or double-vessel disease is also indicated for both symptomatic and prognostic reasons even in the absence of LV dysfunction.18,19 Coronary revascularization has never been shown to confer a survival benefit in patients with single-vessel disease. This is also true for isolated proximal LAD stenoses. Yusuf’s metanalysis23 showing a survival benefit from surgery in patients with LAD involvement must be interpreted with the notion that this result was obtained in a cohort having predominantly multivessel disease. More recently, the randomized MASS (Medicine, Angioplasty or Surgery Study) trial compared lone medical treatment with plain balloon angioplasty or CABG in 214 patients with symptomatic, isolated, high-grade stenosis of the LAD.27 During a 5-year follow-up, there was no appreciable difference between the three treatment arms with regard to either death or MI. Although the power to detect small differences in event rates was low in MASS, its results are consistent with the current judgment that there is no prognostic indication for coronary revascularization in stable single-vessel disease. No study ever demonstrated that, in patients with stable angina, the risk of subsequent MI can be reduced by either bypass surgery or PCI. The degree of stenosis is a notoriously poor predictor of subsequent events. Although the risk of subsequent MI is higher with high-grade stenoses than with low-grade stenoses, the latter are far more frequent than the former. Thus the majority of infarctions are triggered by low-grade stenoses. Despite recent advances,28,29 our current means of identifying vulnerable plaques are limited.
Technical Feasibility
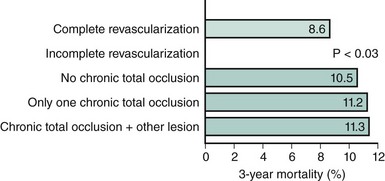
Apart from the extent and distribution of coronary artery disease, the probability of achieving complete revascularization is an important criterion for the choice of the most appropriate revascularization strategy. In CABG, a number of studies have demonstrated that patients who are completely revascularized have better long-term outcomes than those with incomplete revascularization.30 The same is also true for PCI. Several studies from the pre-stent era have confirmed better long-term outcomes after complete revascularization than after an incomplete procedure.31,32 The reasons for not treating all diseased vessels may include technical obstacles such as heavy calcification, tortuous vessels or chronic total occlusions, the presence of serious concomitant disease, or the intention to treat only the “culprit lesion” thought to be responsible for the patient’s symptoms. A recent analysis of a total of 21,945 stent patients from New York State’s Percutaneous Coronary Interventions Reporting System assessed the issue of incomplete revascularization with current practices of coronary revascularization. A follow-up period of 3 years was reported.33 In this registry, 68.9% of the stent patients were incompletely revascularized. After adjustment for comorbidities and other baseline characteristics associated with increased risk, incompletely revascularized patients were significantly more likely to die at any time than completely revascularized patients (adjusted hazard ratio = 1.15; 95% confidence interval, 1.01 to 1.30). The risk associated with incomplete revascularization increased with the number of vessels that were not revascularized and was higher with nonrevascularized chronic total occlusions than in nonrevascularized nonocclusive lesions. Incompletely revascularized patients with total occlusions and ≥2 nonrevascularized vessels were at the highest risk compared with completely revascularized patients (hazard ratio = 1.36; 95% confidence interval, 1.12 to 1.66) (Figure 2-3). Given the major impact of the extent of revascularization on long-term survival, consideration must be given to the likelihood of achieving complete revascularization. When PCI is unlikely to achieve complete revascularization, surgery may offer better prospects. Yet this may not always be the case. In some instances, poor target vessels for CABG may be treated by PCI with higher chances of success.
 Prognostic Indication for Revascularization: PCI Versus CABG
Prognostic Indication for Revascularization: PCI Versus CABG
Multivessel Disease
From the late 1980s to the early 1990s, several studies compared plain balloon angioplasty with CABG. Among them were three larger trials—RITA (n = 1011), CABRI (n = 1154), and BARI (n = 1829)—and three smaller trials—GABI (n = 358), EAST (n = 392), and the Toulouse monocentric study (n = 152). In each of these trials survival after PCI versus CABG was similar, as was the incidence of Q-wave MI, but repeat revascularization was more frequently needed after PCI. However, Hoffmann et al., in a metanalysis based on data extracted from the literature, showed a significant survival benefit from surgery as compared with PCI of 3% absolute at 5 years and of 4% absolute at 8 years.34 The results of the early studies antedating the stent era are, of course, not reflective of the current practice of coronary revascularization. Since the early studies, major advances have been achieved in PCI, CABG, and medical treatment, including coronary stents, effective antiplatelet therapy, the use of arterial conduits up to complete arterial revascularization, and vigorous pharmacological risk-factor modification. For these reasons, the results of randomized trials performed in the pre-stent era cannot be transferred to current practice.
Lessons from Studies with Bare Metal Stents
Randomized Studies
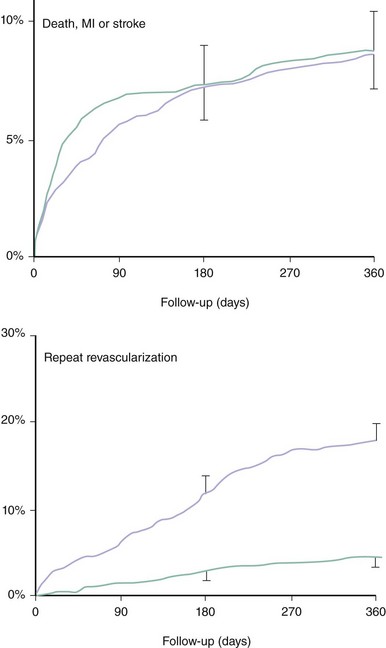
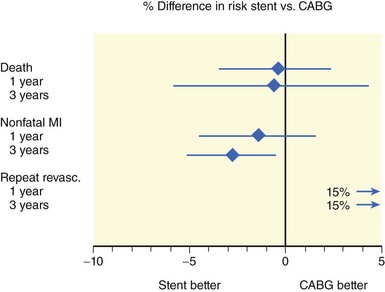
Five randomized trials compared stenting with CABG for multivessel disease: ARTS,35,36 SoS,37 ERACI-2,38,39 MASS-2,40 and AWESOME.41 Four major studies were incorporated in a metanalysis based on individual patient data, which confirmed the results of the majority of the individual studies.42 This metanalysis comprised ARTS, SoS, ERACI-2, and MASS-2 but excluded AWESOME, because the high-risk characteristics of the patients in this last trial were clearly different from those of the patient population of the four other trials. This metanalysis confirmed that PCI with stent placement was associated with a similar 1-year incidence of death, MI, or stroke as CABG (Figure 2-4). Nevertheless, the need for repeat revascularization was considerably higher after PCI, although the observed gap with CABG surgery has narrowed from the approximately 30% reported in the pre-stent era to approximately 14% (Figure 2-4). As compared with PCI, CABG was associated with a slightly lower frequency of recurrent angina (77% vs. 82%; P = 0.002). Another metanalysis based on aggregate data from ARTS, SoS, ERACI-2, and SIMA, a study on isolated proximal LAD stenosis, extended the analysis to a follow-up of 3 years.34 The point estimates for both the 3-year incidence of death and nonfatal MI were lower after PCI than after CABG. However, a significant difference was found only for nonfatal MI (Figure 2-5). Moreover, this metanalysis confirmed that the 1-year incidence of repeat intervention was 15% absolute higher after PCI than after CABG but did not demonstrate any significant further changes from 1 to 3 years. For ARTS, the largest trial comparing PCI with CABG for the treatment of multivessel disease,35,36 5-year results are available. ARTS included a total of 1,205 patients with at least two de novo lesions located in different vessels and territories not including the left main coronary artery. In this study, 600 patients were randomly assigned to stenting and 605 to bypass surgery; 67% of the patients had a double-vessel disease and 32% triple-vessel disease. At 5 years, the incidence of death was 8% in the stent group versus 7.6% in the CABG group (relative risk 1.05 [95% confidence interval, 0.71 to 1.55], P = 0.83). Likewise, there was no significant difference in cerebrovascular accident (3.8% vs. 3.5%, relative risk 1.10 [95% confidence interval, 0.62 to 1.97], P = 0.76), Q-wave MI (6.7% vs. 5.6%, relative risk 1.19 [95% confidence interval, 0.76 to 1.85], P = 0.47), non-Q-wave MI (1.8% vs. 0.8%, relative risk 2.22 [95% confidence interval, 0.78 to 6.35], P = 0.14), or the composite thereof (18.2% vs. 14.9%, relative risk 1.22 [95% confidence interval 0.95 to 1.58], P = 0.14). There was, however, a significant difference in the incidence of repeat revascularization (30.3% vs. 8.8%, relative risk 3.46 [95% confidence interval, 2.61 to 4.60], P < 0.001). In the stent group, 10.5% of the revascularizations involved CABG, whereas it was 1.2% in the CABG group. In summary, the 5-year outcome with respect to the serious endpoints death, MI, and cerebrovascular accident with the surgical and nonsurgical approaches was similar. With the primarily catheter-based approach, there was a 90% chance of avoiding CABG during the subsequent 5 years, with a similar outcome with respect to death, cerebrovascular accident, and MI as with the surgical approach but at the expense of a 20% higher incidence of repeat catheter interventions. Consistent with the long-term results of ARTS, the recently published 10-year results of MASS-2 showed no significant survival benefit of CABG over PCI (hazard ratio [95%-confidence limit]: 1.03 [0.69–1.53], P = 0.88), but a substantially increased need for repeat interventions with PCI versus CABG (hazard ratio [95%-confidence limit]: 3.71 [1.82–7.52], P < 0.0001).43