INDICATIONS
Current indications for stent placement approved by U.S. Food and Drug Administration include the palliation of esophageal obstruction and tracheoesophageal fistulas secondary to malignancy. Other less common applications for esophageal stent placement include dysphagia secondary to external compression by benign neoplasms, benign strictures, and esophageal leak or perforation.
TABLE 34.1 Available Modalities for the Palliation of Dysphagia from Esophageal Cancer

The most common indication for esophageal stent insertion is relief of dysphagia in the setting of unresectable esophageal cancer. The majority of newly diagnosed patients with esophageal cancer have advanced disease at the time of diagnosis, with dismal 5-year survival rates of less than 20%.3 Palliation of dysphagia, therefore, becomes a paramount component of care in this setting. Stents provide safe and expeditious relief of dysphagia, thereby enhancing the patient’s nutritional status and quality of life. Dysphagia can also result from obstructing lesions of the esophagus related to adjacent lung cancer or mediastinal lymphadenopathy due to extrinsic compression. Benign, refractory strictures related to peptic ulcer disease or prior caustic injury can be treated with stent insertion in selected cases. Stent placement may be either temporary or permanent, depending upon the clinical circumstances. In the setting of malignancy, temporary stents represent useful adjuncts prior to surgical resection by relieving dysphagia and enhancing nutrition. However, we have noted, as have others, that expandable metal stents deployed prior to chemotherapy and radiation therapy have been associated with significant esophageal fibrosis, and even perforation, that can cause significant technical difficulties during subsequent surgical resection. Thus, we recommend avoiding esophageal stents in the setting of planned chemotherapy and radiation therapy in the neoadjuvant setting. Temporary stent placement has been successful when used selectively in the management of perforations, leaks, and benign strictures.
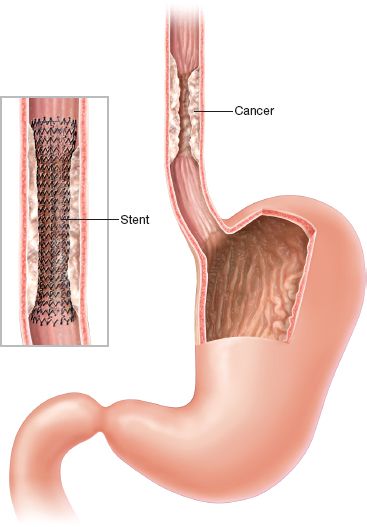
Figure 34.1 Esophageal stent insertion for malignant disease.
Special Considerations
Bridge to surgery: Esophageal stents can be utilized as a temporary measure to enhance oral intake in preparation for definitive surgical resection. Potential limitations of the use of partially covered and uncovered nonremovable stents in this setting include the finding of increased periesophageal desmoplastic reaction that can obscure tissue planes and make the planned surgery difficult. This is especially notable in the setting of neoadjuvant therapy (chemotherapy, chemoradiation). Some authors have suggested that the concomitant use of esophageal stents during chemotherapy and radiation therapy may be associated with an increased risk of complications including migration, bleeding, and fistulization.4,5 Fully covered stents can be placed preoperatively and removed prior to surgery during the course of neoadjuvant therapy to minimize the risk of these delayed complications.6 In our experience, the combination of chemotherapy and radiation has been the most problematic in the setting of an expandable metal stent in the esophagus.
Proximal esophageal cancer: Esophageal stents can be employed in the proximal esophagus to relieve esophageal obstruction and control fistulae. The use of stents in this setting can be limited due to patient intolerance secondary to pain and globus sensation, as well as airway compression.7 Stent positioning distal to the cricopharyngeus is critical in minimizing the risk of these symptoms. Performing flexible bronchoscopy before placing a stent for proximal obstructing esophageal cancers can be helpful in minimizing complications of airway compression. In some cases, we place a guidewire and a Savary dilator of the approximate size of the planned esophageal stent, then flexible bronchoscopy can be performed to assess airway compression. If airway compression is present, it may be prudent to consider an alternative mode of palliating the proximal esophageal obstruction, such as PDT, or even a smaller stent. In some cases, we have placed an airway stent at the same setting to maintain airway patency.
Antireflux valves: For tumors involving the gastroesophageal (GE) junction, stent placement can lead to the development of severe reflux symptoms due to compromise of the lower esophageal sphincter valve mechanism. Several stent modifications have been developed in an attempt to create an antireflux valve mechanism. Dua et al.8 reported a significant improvement in reflux in patients treated with a modified Z stent containing a windsock valve. Results from randomized studies, however, are mixed. Laasch et al.9 demonstrated a significant reduction (12% vs. 96%, p < 0.001) in reflux among 50 patients receiving either the Dua-modified Z stent compared with those treated with the Flamingo Wallstent. In another study evaluating the effectiveness of an S-shaped antireflux valve (Dostent, MI Tech Co. Ltd., Incheon, South Korea), the fraction of time with an intraesophageal pH < 4 was 3% in the group with the antireflux stent and 15% in the group with self-expanding metallic stents (SEMS).10 Other randomized trials, however, have failed to demonstrate improvement in reflux symptoms or objective measures of gastroesophageal reflux during pH testing with the use of antireflux valves.11,12 As a result of these disparate findings, antireflux valves are not routinely used during esophageal stenting in the setting of malignancy.
Drug-eluting stents: Drug-eluting stents have been developed in an attempt to minimize tissue ingrowth in partially covered stents.13 This type of stent is not currently available for use in humans.
Biodegradable stents: Stents composed of biodegradable material (knitted poly-L-lactic acid monofilaments) have been used to prevent stricture formation following large area endoscopic mucosal resection.14 Another biodegradable stent model (Ella-CS) has been studied in Europe for the treatment of benign disease.15 Biodegradable stents have the advantage of potentially reducing the need for repeat endoscopy to accomplish stent removal.
Stent Design
Prior to 1990, virtually all esophageal stents were composed of polyvinyl plastic or rubber. These early stents were cumbersome and very difficult to place, requiring insertion at the time of open surgery or during rigid endoscopy. With the introduction of SEMS, the use of rigid stent prostheses fell out of favor. SEMS are easier to deploy, achieve a wider luminal diameter and are associated with a reduced periprocedural complication rate (including mortality).16 Although the cost of SEMS is greater than rigid prostheses, the need for repeat interventions is less, leading to an overall reduced cost over the limited expected lifetime of a patient with advanced esophageal cancer.17
Most SEMS that are currently available today are constructed with Nitinol (a composite of nickel and titanium), which is a highly elastic alloy with intrinsic properties of elasticity and shape memory that allow conformation to varying degrees of stenosis and angulation. The application of these pliable constructs allows the use of a lower profile delivery system while achieving efficient transmission of adequate radial force upon stent expansion. Initially, all SEMS were uncovered (Fig. 34.2A). Examples of uncovered stents include the Ultraflex uncovered esophageal stent and the Microvasive Wallstent I (Boston Scientific, Natick, MA). Uncovered stents expand radially and incorporate into the wall of the esophagus with time.18 This incorporation effect dramatically reduces the potential for stent migration seen with rigid plastic prostheses and with covered metal stents. However, the open spaces within the interstices of the uncovered metallic stent permit the ingrowth of granulation tissue or tumor leading to recurrent symptoms of dysphagia in 13% to 26% of cases.19
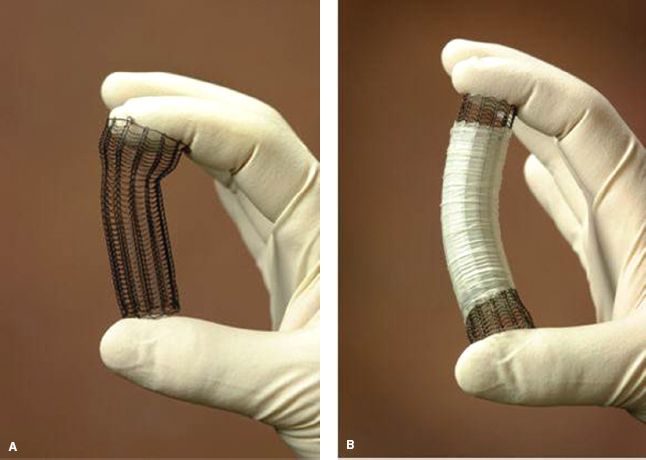
Figure 34.2 Uncovered (A) and Covered (B) expanding metallic stent. (From: Perry Y, Luketich JD. The use of esophageal stents. Cameron JL, ed. Current Surgical Therapy. 8th ed. Philadelphia, PA: Mosby; 2004:49–55.)
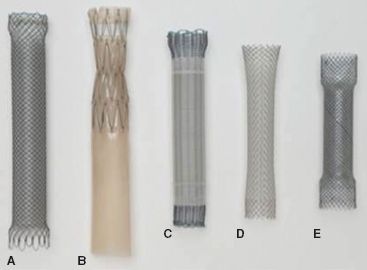
Figure 34.3 Partially covered self-expanding metallic stents. (A) Wallflex, (B) Z stent with Dua anti-reflux valve, (C) Ultraflex, (D) Wallstent, (E) Evolution. (From: Schembre D. Advances in esophageal stenting: The evolution of fully covered stents for malignant and benign disease. Adv Ther. 2010;27(7): 413–425.)
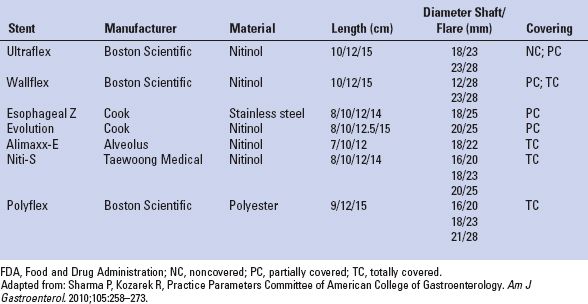
To minimize the occurrence of tumor ingrowth and the development of esophageal erosions or tracheoesophageal fistulas, stents were developed that were partially covered with silicone, polyurethane, or other polymers (Fig. 34.2B). Current designs maintain a 1- to 1.5-cm margin of exposed wire struts on the proximal and distal flanges of the stent to optimize stent purchase and permit integration into the esophageal wall (Fig. 34.3). The use of a covered stent has been shown to significantly decrease the severity of tumor ingrowth and consequent dysphagia, but is associated with a slightly higher migration rate. In a large retrospective analysis of 152 patients who underwent either uncovered (n = 54) or covered (n = 98) SEMS placement, uncovered stents were associated with reduced migration (0% vs. 10 %, p = 0.04) but were also associated with a significantly increased rate of tissue ingrowth (100% vs. 53%, p < 0.0001) and a markedly higher restenosis rate resulting in recurrent dysphagia (37% vs. 8%, p < 0.0001).20 In a prospective, randomized comparison of 62 patients with inoperable GE junction tumors treated with either covered or uncovered stents, a significantly higher reintervention rate was noted in patients with uncovered stents compared with covered stents (27% vs. 0%). There was comparable relief of dysphagia between groups. Tumor ingrowth or granulation tissue was more commonly encountered in the uncovered stent group (30% vs. 3%, p = 0.005). There was no difference in survival noted between groups.19 Although this new generation of covered stents dramatically reduced tumor ingrowth, mucosal hyperplasia/hypertrophic granulation tissue still develops at the uncovered proximal and distal margins of the stent leading to recurrent obstruction. Incorporated stents may thus be difficult or impossible to remove. Examples of currently available covered stents include the Ultraflex Covered Esophageal Stent (Microvasive Endoscopy/Boston Scientific Corp., Natick, MA), the Alimaxx-E (Alveolus, Inc, Charlotte, NC), the Flamingo Wallstent (Schneider AG, Bulach, Switzerland), the Gianturco-Z stent (Wilson-Cook Europe AIS, Bjæverskov, Denmark), the Song stent (Sooho Meditech, Seoul, Korea), and the Esophacoil stent (Medtronic/InStent Inc., Eden Prairie, MN) (Fig. 34.3; Table 34.2). The most commonly used partially covered stent in the United States is the Ultraflex stent. The Ultraflex stent is made of nitinol and is covered along its midsection by a polyurethane coat. There is approximately 1.5 cm of uncovered nitinol mesh at the proximal and distal ends of the stent. The stent is positioned over a guidewire and is deployed with the release of a slip-knotted binding string. Proximal and distal drawstrings allow adjustment of stent position following deployment. The Wallflex stent is also composed of nitinol and may be partially covered or completely covered. Its deployment mechanism allows recapture of the stent up to approximately 75% deployment. Similar to the Ultraflex stent, a proximal purse-string suture can be utilized to adjust stent position. The length of the proximal and distal flares is longer than the Ultraflex stent in the hope of reducing stent migration. The Z stents, also known as Gianturco-Rösch Z stents (Cook Endoscopy, Winston-Salem, NC), are constructed from stainless steel woven in an interlocking “Z” configuration. A modification of the standard configuration involves the addition of a windsock extension at the distal end of the stent which acts as a one-way valve, thus reducing the risk of reflux (Dua antireflux system). Cook has also produced the Evolution partially covered stent that is deployed with a gun-like deployment mechanism. Similar to the Wallflex stent, this mechanism allows for recapture prior to complete deployment.
TABLE 34.2 FDA-approved Self-expanding Stents Currently Available in the United States
To date, prospective, randomized trials have not demonstrated any significant advantage of one design of covered SEMS over another in terms of technical success, morbidity, relief of dysphagia, or survival in the setting of malignant obstruction.21,22 In a direct, prospective, randomized comparison of the Ultraflex, Wallstent, and Gianturco Z stent, there was no significant difference noted in the palliation of dysphagia. The Flamingo Wall stent had the lowest morbidity profile (18%), but this finding did not achieve statistical significance when compared with the Ultraflex stent (24%) and the Gianturco Z stent (36%).21 Similar findings were obtained in another study comparing these same three stent types, with equivalent relief of dysphagia. In this study, the Gianturco Z stent was associated with a significantly higher complication rate compared with the Ultraflex and Flamingo Wallstent.23 The Gianturco Z stent is no longer available in the United States.
Completely covered plastic stents were introduced into the market in 2001, and expanded the number of applications for stent usage, including benign conditions not readily treatable by SEMS due to their erosive tendencies. These stents lack the ingrowth properties of metallic stents and are removable. Plastic stents are limited, however, by increased migration rates due to the mechanical characteristics of the plastic coating and decreased purchase upon the esophageal wall. Plastic stents do exert greater radial force than SEMS, which can lead to migration by “squirting” either proximally or distally with respect to the narrowed segment. The increased radial force can also lead to discomfort or pain. Some of the plastic stent delivery systems are stiff and bulky, making deployment difficult in severely narrowed segments. The Polyflex stent (Boston Scientific, Natick, MA) is composed of a polyester mesh coated with an outer layer of silicone. It is deployed via a push-and-release technique after being loaded into the delivery system. The proximal portion of the stent is flared to help minimize the risk of migration. The Alimaxx-E stent is composed of a nitinol core structure completely covered with silicone. The outer surface of the stent has small struts that help to keep the stent anchored to the esophageal lumen and to prevent migration. This stent can be released over a wire using a trigger-graded release mechanism, and can also be loaded directly onto a pediatric endoscope and deployed under direct visualization without the need for fluoroscopy. Another completely covered stent is the Niti-S stent (Taewoong Medical, Seoul, Korea). This stent has a design that is similar to the Wallflex stent with broad flaring ends. It is currently in limited distribution within the United States (Fig. 34.4).
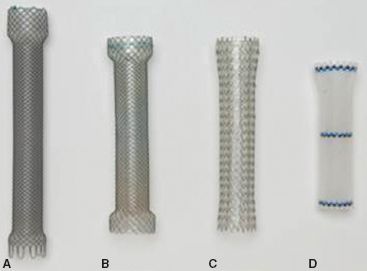
Figure 34.4 Completely covered self-expanding stents. (A) Wallflex, (B) Niti-S,(C) Alimaxx-E, (D) Polyflex. (From: Schembre D. Advances in esophageal stenting: The evolution of fully covered stents for malignant and benign disease. Adv Ther. 2010; 27(7):413–425.)
Early experience with the use of self-expanding plastic stents reveals similar relief of dysphagia compared with SEMS in >98% of patients. The most notable problem with expandable, plastic stents is the issue of stent migration.24 Conigliaro et al.25 documented a 20% stent migration rate (n = 12/60) with seven early migrations and five occurring late. Prospective randomized studies comparing SEMS with self-expanding plastic stents have demonstrated equivalent relief of dysphagia in the setting of esophageal cancer; however, self-expanding plastic stents have been associated with increased difficulty of insertion and complication rates (migration, hemorrhage, food impaction) in comparison with SEMS. In a randomized comparison of 101 patients (Ultraflex = 54; Polyflex = 47) who underwent stent placement for unresectable esophageal carcinoma, success of stent insertion, initial relief of dysphagia, and overall survival were similar between groups. Self-expanding plastic stents were, however, associated with a significantly higher complication rate (hyperplastic overgrowth, migration, food impaction) compared with SEMS (odds ratio = 2.3; 95% confidence interval: 1.2 to 4.4).26 In another prospective, randomized comparison of patients undergoing stent placement for malignant dysphagia (Ultraflex = 42, Niti-S = 42, Polyflex = 41), relief of dysphagia, and overall survival was similar between groups. There was an increased rate of tissue ingrowth in the partially covered SEMS (Ultraflex) group, though this did not attain statistical significance. Self-expanding plastic stents were associated with an increased rate of stent migration (29%, p = 0.01) as well as increased technical difficulties in stent placement (p = 0.008) when compared with SEMS.27
The most recent generation of stents highlights fully covered SEMS that are designed to overcome the limitations of partially covered SEMS and self-expanding plastic stents (Fig. 34.4). These stents may promote less granulation tissue and associated stenosis, and may be removable after several weeks. However, an increased migration rate still may be expected as a result.28 Published data on the performance of fully covered stents are awaited.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Preoperative assessment of clinical symptoms should be objectively recorded. Dysphagia scores are utilized to gauge the degree of symptomatic esophageal obstruction. The following scoring system is commonly employed: 0, tolerating a regular diet, no dysphagia; 1, difficulty with solids; 2, difficulty with soft foods; 3, difficulty with liquids; 4, difficulty managing saliva. Careful preoperative radiographic assessment is imperative in planning the optimal approach. Barium esophagography provides a roadmap of the esophagus, and allows assessment of the extent of obstruction, the length of esophageal involvement, and the presence of other anatomic abnormalities such as leak, perforation, or fistula. Computed tomography and PET scanning are critical in the setting of malignancy in helping to stage the extent of disease. Esophagogastroduodenoscopy (EGD) allows confirmation of the underlying pathology, a real-time assessment of the extent of disease, and whether the obstruction is intrinsic or extrinsic in nature. Flexible bronchoscopy is a useful adjunct in assessing patients with esophageal cancers of the proximal-mid esophagus and in those with suspected tracheoesophageal fistulas.
 TECHNIQUE
TECHNIQUE
Esophageal stents may be inserted endoscopically under conscious sedation or via a general anesthetic. The authors prefer to employ a general anesthetic in most cases in an effort to minimize the risk of aspiration and optimize stent positioning. During EGD, the following features of the case are assessed: (1) degree of esophageal narrowing, (2) location and length of esophageal involvement, and (3) the integrity of surrounding esophageal tissues. On occasion, significant narrowing may prevent the safe passage of the endoscope, and gentle dilation is performed to enable advancement of the scope beyond the distal extent of the lesion. Care should be taken to minimize the extent of dilation if anticipating the need for stent insertion. Overdilation of a malignant stricture might decrease stent purchase after deployment, leading to a higher risk of stent migration. Once the obstructing lesion has been assessed, the proximal and distal extent of the lesion is mapped with radio-opaque markers that are placed on the skin of the chest wall (Fig. 34.5). The scope is then advanced into the duodenum, and a guidewire is inserted. The scope is withdrawn, and the distance between the two external markers is measured. This distance is used to estimate the length of the stent chosen to cover the lesion. Overall stent length will need to extend 1 to 2 cm above and below the marked interval to ensure complete coverage and to optimize stent position and minimize the risk of the end of the stent “crimping” and not fully deploying if it is too near the obstructive tumor. The stent delivery system (Fig. 34.6) is then advanced over the wire under fluoroscopic guidance. The stent is identified by radio-opaque proximal and distal markers that are aligned with the previously placed skin markers. Once adequate position is confirmed, the stent is deployed under fluoroscopic guidance. Delivery systems may employ a proximal release or distal release technique. Slight adjustments to stent position can be made during deployment to optimize stent position. Following deployment, the delivery system and guidewire are removed, and repeat endoscopy is performed to assess the adequacy of stent position and expansion (Fig. 34.7). Stents should be slightly oversized to permit increased pressure of the stent against the esophageal wall, so as to minimize the risk of stent migration. Care should be taken not to select a diameter that is too large, which can lead to pain and incomplete stent expansion with residual infolding of the stent that will partially obstruct the stent lumen. Appropriate deployment will result in a stent that is well expanded with good purchase along the esophageal wall. A slight indentation at the level of obstruction is evident on fluoroscopy (Fig. 34.8). Ideally, there should be no gap between the esophageal stent wall and proximal stent lumen. Areas of incomplete expansion can be augmented with the assistance of a balloon dilator. Most stents are equipped with proximal and distal purse strings that permit stent repositioning as required following deployment. Utilizing careful technique, success rates of 80% to 90% for stent deployment can be expected.
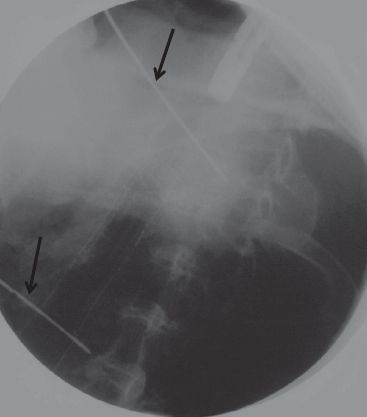
Figure 34.5 Deployment of esophageal stent. Note the proximal and distal skin markers that delineate the extent of obstructing tumor (arrows). Fluoroscopy demonstrates good stent position and expansion. (From: Perry Y, Luketich JD. The use of esophageal stents. Cameron JL, ed. Current Surgical Therapy. 8th ed. Philadelphia, PA: Mosby, 2004: 49–55.)



