Heart rate, blood pressure, and vascular tone, as well as ventilator drive, respiratory rate, and breathing pattern, are, at least in part, under the control of specific reflexes. These reflexes are mediated by a complex network of baroreceptors and chemoreceptors in the arterial system of the carotids, aorta, and left heart, including receptors in the left atrium, the ventricle, and the coronary arteries; irritants in the upper airways and stretch receptors in the lower airways; juxtacapillary-located nonmyelinated fibers in the alveoli and in the bronchial arterial system; and muscle spindles that evoke changes in the membrane potential upon alteration of sarcolemmal tension. Some of these reflexes, usually named after the first individual to describe them, have spread as eponyms into propaedeutic education and clinical work. Because these euphonic eponyms are enigmatic to most clinicians today, this article is intended to provide a short overview of these reflexes, including the historical context of their describers. As evidenced by their clinical implications, the eponyms discussed are revealed to be more than curiosities taught during undergraduate medical education.
Heart rate, blood pressure, and vascular tone, as well as ventilator drive, respiratory rate, and breathing pattern, are, at least in part, under the control of specific reflexes. These reflexes are mediated by a complex network of baroreceptors and chemoreceptors in the arterial system of the carotids, aorta, and left heart, including receptors in left atrium, the ventricle, and the coronary arteries; irritants in the upper and stretch receptors in the lower airways; juxtacapillary-located nonmyelinated fibers in the alveoli and in the bronchial arterial system; and muscle spindles that evoke changes in the membrane potential upon alteration of sarcolemmal tension (summarized in West et al ). Some of these reflexes, usually named after the first individual to describe them, have spread as eponyms into propaedeutic education and clinical work. It is not within the scope of this article to review all reflexive cardiopulmonary responses, and thus, many interesting reflexes such as the carotid baroreflex or the diving reflex are not discussed. However, because the euphonic eponyms listed in Figure 1 and Table 1 are enigmatic to most clinicians today, this article provides a short overview of these reflexes, including the historical context of their describers. As evidenced by their clinical implications, the eponyms discussed are revealed to be more than curiosities taught during undergraduate medical education.
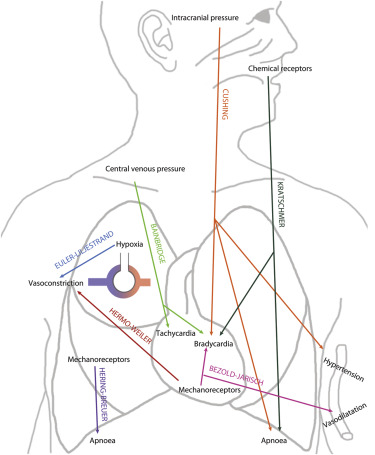
| Eponym | First Description | Regulation | Physiology | Clinical Implication |
|---|---|---|---|---|
| Valsalva | 1704 | Changes of intrathoracic pressure, altered venous return, sympathetic activity | Hyper-/hypotension, brady-/tachycardia (see text) | Altered response in congestive heart failure; useful tool in auscultation of systolic cardiac murmurs |
| Bezold-Jarisch | 1867 | Left ventricular mechanoreceptors | Bradycardia, hypotension | Exertional syncopal events in aortic stenosis |
| Hering-Breuer | 1868 | Pulmonary mechanoreceptors | Regulator of tidal volumes | Postnatal prevention of lung overinflation |
| Kratschmer | 1870 | Mucosal chemoreceptors of the airways | Apnea, Bradycardia | Complicates surgical interventions in the cranio-maxillary region |
| Cushing | 1902 | Sympathetic response following cerebral ischemia | Hypertension, bradycardia, irregular breathing pattern | Alarming signs in intracranial hypertension |
| Bainbridge | 1915 | Cardiac stretch receptors, vagal nerve | Tachycardia upon increased venous return | |
| Euler-Liljestrand | 1946 | Hypoxia induced alterations of vascular smooth muscle calcium levels | Hypoxic pulmonary arterial vasoconstriction | Pulmonary hypertension in hypoxemic lung disease |
| Hermo-Weiler | 1998 | Left atrial mechanoreceptors | Precapillary pulmonary vasoconstriction | Reactive elevation of transpulmonary pressure gradient |
Valsalva Reflex
Antonio Maria Valsalva (1666 to 1723) was a professor of anatomy at the University of Bologna and President of the Academy of Sciences. In 1704, he published his work on the anatomy and physiology of the ear, which was considered for years to be the standard reference on the topic. The Valsalva maneuver (a sustained forced expiratory effort against the resistance of a closed nose or glottis) was originally intended to clear the Eustachian tube. Translated from the original Latin (which can be assessed online: http://openlibrary.org/books/OL2669149M/De_aure_humana_tractatus ), the maneuver is described thus: “if with occluded mouth and nostrils air is compressed inwardly, this action will extrude sanies from the middle ear [and is] a remedial exercise, to be repeated [and will lead to] extrusion of praeter-natural cerebral matter either via the wound, via the nostrils, via the mouth, or via the auditory meatus … with great benefit.” The role of the maneuver in cardiovascular medicine was first described approximately 150 years later. Details of the historical context have been reviewed by Jellinek. The hemodynamic alterations observed during the Valsalva maneuver can be grouped in 4 phases as shown in Figure 2 . In phase I, forced expiration against obstructed airways raises both the intrathoracic pressure, leading to an increased systolic blood pressure due to compression of the aorta. Concomitantly, venous return is decreased, resulting in lower cardiac output and thus subsequent fall of systolic blood pressure and reflex increase of heart rate (phase II). In the next phase, release of the respiratory efforts results in decrease of intrathoracic pressure with blood pooling within the pulmonary vasculature, which leads to a further decrease in cardiac preload and systolic blood pressure (phase III). The last phase is characterized by an overshoot of blood pressure and reflex bradycardia resulting from increased cardiac output (phase IV).
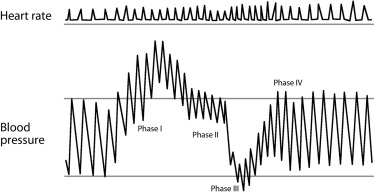
The most common clinical indication for the Valsalva maneuver is the differentiation of systolic heart murmurs: most murmurs decrease in length and intensity during the maneuver. Two exceptions are the systolic murmurs of hypertrophic obstructive cardiomyopathy (becoming louder during phase II because of the decrease in preload in the left ventricle) and those of mitral valve prolapse (becoming longer). The release phase (phase IV) can also be used during auscultation to differentiate murmurs originating from the right side of the heart (early return to the normal intensity, within 1 to 2 beats) from murmurs from the left side (delayed return of intensity, after 5 to 6 beats). Aberrant reactions to the Valsalva maneuver are also observed in cardiac disorders such as congestive left heart failure. The “absent overshoot” of systolic blood pressure during phase IV indicates decreased systolic dysfunction and is observed in patients with less severe heart failure. The “square wave” response, seen in more severe heart failure, is characterized by a lack of decrease in blood pressure during phase II. In patients with increased left ventricular end-diastolic volume, the reduced left ventricular preload during Valsalva maneuver due to diminished right-sided venous return does not affect the stroke volume. Therefore, in patients with increased left ventricular preload, the reflex tachycardia observed during phase II is lacking. After correction of the preload by diuretics and/or nitrates, the response to the Valsalva maneuver normalizes. The Valsalva response is also altered in diseases of the autonomic nervous system (reduced heart rate variability during Valsalva maneuver suggests impaired autonomic function) and implicated in the mechanism of situational syncope. Last but not least, the Valsalva maneuver causes a transient increase in vagal tone: tachyarrhythmias that depend on the AV node for continuation can terminate or slow with this maneuver.
Bezold-Jarisch Reflex
In 1867, 2 German physiologists, Albert von Bezold (1836 to 1868) and Ludwig Hirt (1844 to 1907) observed that intravenous injection of alkaloids from the Veratrum genus (perennial plants from the lily family; Hollman ) caused profound decreases in both blood pressure and heart rate with concomitant apnea. Seventy years later, Adolf Jarisch Jr. (1891 to 1965) at the University of Innsbruck confirmed these observations. Jarisch performed experiments with cats in which veratridine was injected before and after interruption of the cardiac branches of the vagal nerve. He postulated that the depressor effect was reflexive in origin and argued that the receptors responsible for this effect had to be located in the ventricle of the heart. Dawes and coworkers showed in 1947 that the apnea was due to a mechanism separate from the one mediating hemodynamic changes. Since these experiments were conducted, the term “Bezold-Jarisch reflex” (BJR) refers to bradycardia, vasodilation, and hypotension resulting from the stimulation of cardiac receptors. The cardioinhibitory receptors in the ventricular wall can be activated by multiple stimuli, which all use the vagal nerve as an afferent pathway. Vasodilatation and hypotension then probably result from significant adrenergic withdrawal.
The physiologic role of the BJR is probably related to blood pressure regulation, and activation of this system has been associated with several pathophysiologic conditions including hypovolemia, myocardial infarction, and reperfusion of coronary arteries. The most important clinical implication of the BJR is probably exertional syncope in severe aortic stenosis when activation of baroreceptors and type C mechanoreceptor fibers within the left ventricular wall prevent reflex vasoconstriction of resting extremities. It has also been postulated that the BJR could explain the mechanism of neurocardiogenic syncope, probably together with the Valsalva maneuver, and cardioinhibitory responses observed during anesthesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


