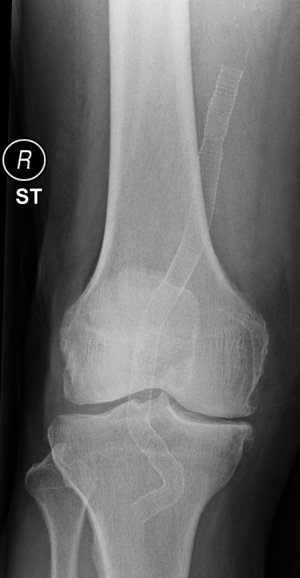
A radiograph of the knee is performed before discharge as a reference for the future detection of graft migration, disconnection, or stent fractures. Duplex ultrasound is particularly suited to detect both endoleaks and stenoses and to evaluate the diameter of the PAA. Our protocol includes follow-up at 6 weeks, 6 months, 1 year, and then yearly with physical examination, duplex ultrasound, ankle-to-brachial index, and knee radiographs with the knee extension (anteroposterior and lateral view) and with the knee in 90 degrees of flexion (lateral view only) (Figures 1 and 2).
Endovascular Treatment of Popliteal Artery Aneurysms
Endovascular Procedure and Follow-Up
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine