Chapter 20 Endovascular Treatment of Peripheral Artery Disease
The concept of nonsurgical catheter-based peripheral vascular revascularization was first described by Charles Dotter1 and further advanced with the development of balloon dilation catheters by Andreas Gruentzig.2 Catheter-based revascularization has largely replaced conventional open surgery as the treatment of first choice in selected patients treated for lower-extremity ischemia.3
No single specialty program (cardiology, radiology, or surgery) offered training that satisfied the entire skill set needed to perform peripheral endovascular intervention (Table 20-1). Recognition of this unmet need for a trained cadre of clinicians to care for patients with peripheral artery disease (PAD) prompted the development of a core cardiology training symposium (COCATS-11) to codify the necessary cardiology fellowship training.4
Table 20-1 Required Skill Elements for Optimal Peripheral Vascular Intervention
| Skill Element | Description |
|---|---|
| Cognitive | Extensive knowledge of vascular disease, including natural history, pathophysiology, diagnostic methods, and treatment alternatives |
| Technical | Competence in both diagnostic angiography and interventional techniques, such as use and selection of balloons, guidewires, stents, and emboli protection devices |
| Clinical | Ability to manage inpatients, interpret laboratory tests, obtain informed consent, assess risk/benefit ratio, and admitting privileges |
Patient and Lesion Selection Criteria
Indications
Anatomical and Functional Criteria
Patient selection for catheter-based vascular intervention depends upon both anatomical and functional criteria (Table 20-2). Anatomical lesion criteria include ability to gain vascular access, a reasonable likelihood of crossing the lesion with a guidewire, and the expectation that a therapeutic catheter can be advanced across the target lesion (Fig. 20-1). A strategy of “provisional” (bailout) stenting, or use of a stent for a failed balloon dilation attempt (in contrast to “primary” stenting, in which stents are placed with or without balloon predilation), has become the standard of practice for shorter, more discrete lesions. Longer lesions and occlusions are better treated with primary stent placement.3,5–8
Table 20-2 Classification of Peripheral Arterial Disease: Fontaine’s Stages and Rutherford’s Categories
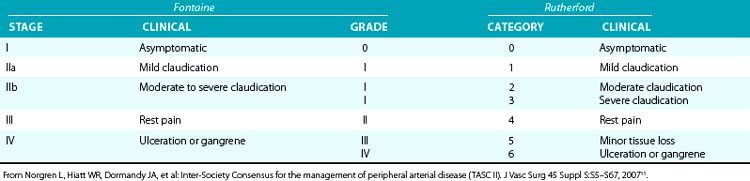
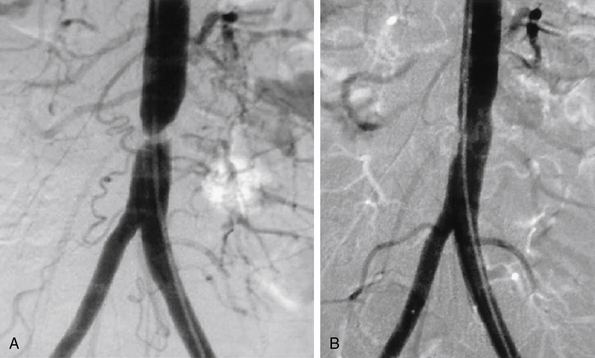
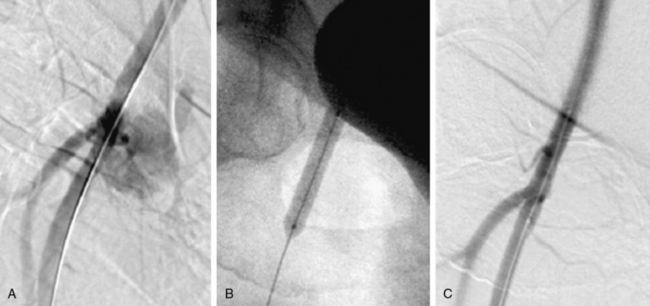
Figure 20-1 A, Tight stenosis of infrarenal aorta amenable to angioplasty. B, Final postangioplasty result.
Availability of endovascular stents (balloon expandable and self-expanding) has significantly extended the anatomical subset of patients who may be considered candidates for peripheral vascular intervention, particularly for longer stenotic lesions and occlusions. The rate-limiting step for nonsurgical revascularization of the aortoiliac vessels is the ability to pass a guidewire across the lesion. Regardless of the balloon dilation result, the option of stent placement offers a reliable and reproducible method to recanalize these large vessels.9
Vascular access site complications following catheter-based procedures often can be treated with percutaneous therapy10 (Fig. 20-2). Patients with hypotension and a high suspicion of bleeding after common femoral artery (CFA) access require urgent diagnostic angiography from the contralateral femoral artery to determine the bleeding site. Rapid identification of the bleeding site may provide an opportunity for lifesaving hemostasis with balloon tamponade.
Patients with CLI or limb-threatening ischemia (gangrene, nonhealing ulcer, or rest pain) are candidates for urgent revascularization. When considering a patient with CLI for revascularization, it is important to remember that multilevel disease (iliac, femoral, and tibial) is likely to be present and that simply improving “inflow” without addressing the more distal vascular lesions or runoff vessels may fail to solve the clinical problem. Patients with CLI (rest pain, nonhealing ulcers, or gangrene) typically have more extensive disease than claudicants and require urgent revascularization to prevent tissue loss3,11 (see Table 20-2).
Prognosis for patients presenting with CLI is poor.12 Those with tobacco abuse and/or diabetes are 10 times more likely to require amputation. Patients with CLI tend to be older, with almost 50% of patients older than 80 years undergoing amputations. Within 3 months of presentation, 12% will require an amputation, and 9% will die; 1-year mortality rate is 22%. Anatomy suitable for endovascular therapy is often present in one or more below-knee vessels. Therapy should be designed to restore pulsatile straight-line flow to the distal part of the limb, with as low a procedural morbidity as possible. The guiding principle is that less blood flow is required to maintain tissue integrity than to heal a wound, so restenosis does not usually result in recurrent CLI unless there has been repeated injury to the limb. Therefore, the emphasis is less on long-term vessel patency and more on amputation-free survival.
The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial was a multicenter randomized trial comparing an initial strategy of balloon angioplasty to open surgery in 452 patients with CLI.13 The primary outcome was time to amputation or death (amputation-free survival). After 6 months, the two treatment strategies did not differ significantly in amputation-free survival. There was no difference between the groups for quality-of-life outcomes, but for the first year of follow-up, costs associated with a surgery-first strategy were higher than for angioplasty. For this reason, the authors concluded that a percutaneous-first strategy was the treatment of choice in patients who are candidates for either surgery or endovascular intervention.
Technical and Procedural Considerations
Preprocedure
General Measures
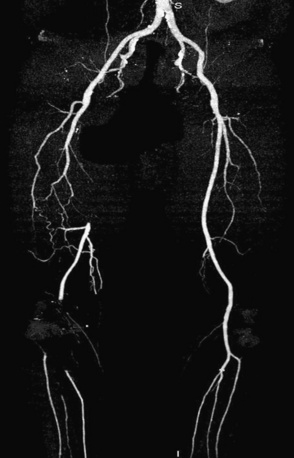
Prior to performing lower-extremity endovascular intervention, it is necessary to objectively determine the patient’s functional status. A history, physical examination, and appropriate noninvasive testing should be obtained prior to planning peripheral endovascular revascularization. If the patient is ambulatory, a rest and exercise ankle-brachial index (ABI) should be measured, and pulse volume recordings (PVR) should be performed. Other noninvasive modalities, such as vascular ultrasound, or alternative imaging modalities, such as magnetic resonance angiography (MRA) or computed tomographic angiography (CTA), may be helpful to resolve conflicting data and are used at the discretion of the physician (Fig. 20-3). When planning lower-extremity revascularization, status of the inflow and outflow vessels relative to the target lesion must be visualized angiographically. This is usually done with invasive diagnostic angiography, but in selected patients, MRA or CTA may be very useful.
Equipment Choices
Stents
The two categories of stents are balloon expandable and self-expanding. Both types may be covered with material. Balloon expandable stents are intended for use within the axial skeleton to protect them from external compression. This generally limits their use to the iliac arteries, but coronary balloon expandable stents are used to salvage failed angioplasty results in below-knee vessels.14,15
Balloon expandable stents can be deployed with more precision than self-expanding stents, although there is some shortening associated with their expansion. Self-expanding stents resist permanent deformation and are elastic. Their flexible nature allows them to delivered in longer lengths, and they will fit themselves to a tapering artery. Self-expanding stents may be made of nitinol or a stainless steel alloy. At this time, there is no evidence that either material is associated with any safety or efficacy advantage.16,17
Clinical Outcomes
Aortoiliac Vessels
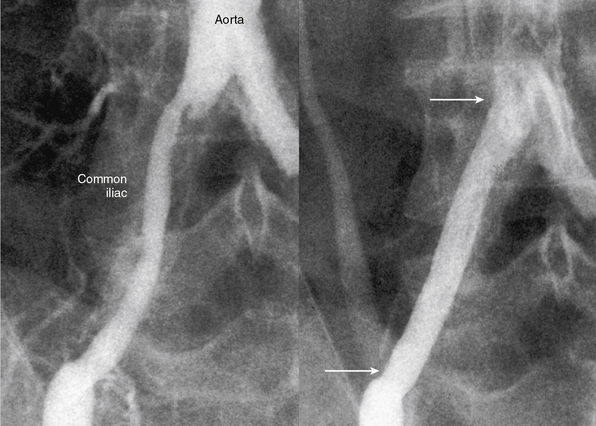
The current best practice, in experienced hands, for aorto-iliac lesions favors an endovascular strategy (Fig. 20-4). This recommendation is based upon the morbidity and mortality associated with major vascular surgery in patients with significant comorbidity, and the excellent outcomes available with current endovascular techniques. In a large single-center registry of 505 iliac stent procedures, the technical success rate was 98%, 8-year primary stent patency rate was 74%, and secondary patency rate was 84%.18 In a 10-year iliac stent patency study, there was no effect of age, diabetes, tobacco smoking, or hypertension on patency.19 Common iliac artery (CIA) lesions had greater long-term patency than external iliac artery (EIA) lesions. Outcomes from another series of 89 consecutive patients with symptomatic occluded iliac arteries demonstrated a 92% success rate for endovascular treatment.20 Increasing severity and complexity of lesions did not significantly alter iliac artery patency rates.
An observational study compared nonrandomized results of iliac artery stenting with surgery in patients with moderately complex lesions.21 There was no difference regarding limb salvage or patient survival out to 5 years, but vessel patency was reduced in limbs treated with stents compared to surgery. A nonrandomized retrospective comparison of endovascular intervention compared to open surgery for complex aortoiliac occlusive lesions found a shorter hospital stay, fewer postprocedural complications, and lower primary patency rates but equivalent secondary patency rates for the endovascular arm.22
There is debate regarding the most efficacious method of endovascular therapy between “provisional stent placement,” which is selective use of stents only when balloon dilation has failed or is suboptimal, and “primary stent placement,” which is the practice of deploying a stent regardless of the balloon result. The Dutch Iliac Stent Trial demonstrated that selective iliac artery stenting achieved an equivalent hemodynamic result compared to primary stenting. Translesional pressure gradients after primary stent placement (5.8 ± 4.7 mmHg) were significantly lower than after balloon angioplasty alone (8.9 ± 6.8 mmHg), but not after provisional stenting (5.9 ± 3.6 mmHg) in the percutaneous transluminal angioplasty (PTA) group.23 The procedural success rate, defined as a postprocedural gradient less than 10 mmHg, revealed no difference between the two treatment strategies, (primary stenting = 81% vs. PTA plus provisional stenting = 89%). By employing a provisional stenting strategy in the iliac artery, stent placement was avoided in 63% of lesions. After 5 years of follow-up, the selective stent placement strategy had greater symptomatic improvement compared with primary stent placement, but there was no difference in patency rates, ABI, and quality of life between groups.24,25
Preferred clinical practice, however, is primary stent placement, and this is supported by a meta-analysis which looked at more than 2000 patients.26 Procedural success was higher in the primary stent group, and there was a 43% reduction in 4-year failures for aortoiliac stent placement compared to balloon angioplasty alone. Advantages of primary stent placement include efficient and reliable vascular reconstruction, minimizing concern over abrupt occlusion. Direct stenting minimizes the technical challenges of determining translesional pressure gradients and the need to administer vasodilator medications. The current American College of Cardiology/American Heart Association (ACC/AHA) guideline document supports primary stenting of the common and EIAs with a class I recommendation (Level of Evidence B).3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree