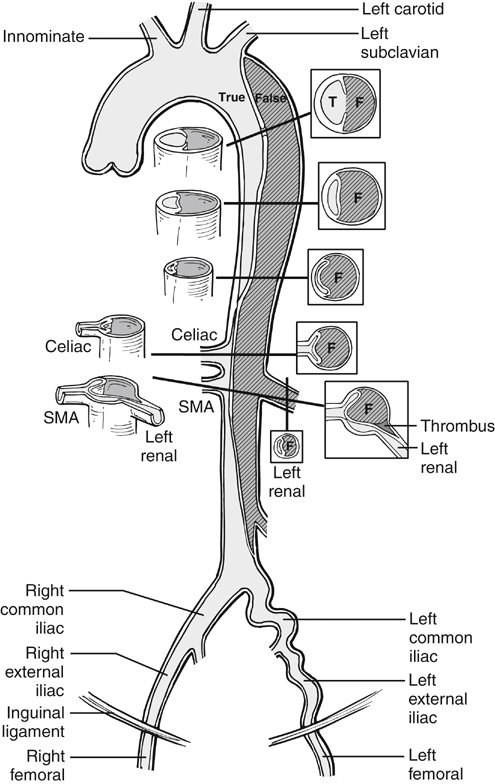
In dynamic obstruction, the true lumen is collapsed, and the flap spares the origin but prolapses across it. Generally several visceral branches are involved simultaneously. Treatment is reducing the flap and providing perfusion to the branches. This is most expeditiously accomplished by deploying an endograft, if the entry tear is anatomically suitable, as in many type B dissections. The endograft excludes the entry tear, diverts blood flow back exclusively into the true lumen, and simultaneously reexpands the true lumen and collapses the false lumen. Placement of an appropriate endograft results in global relief of distal true lumen collapse and dynamic obstruction, and it results in variable relief of static obstruction as well. Following placement of the endograft, perfusion of infradiaphragmatic branches can be assessed and, if indicated, treated by standard clinical and angiographic means (Figures 1 and 2). Three phase II trials of endograft treatment of complicated type B dissections are active in the United States at the time of this writing (W.L. Gore and Associates, Flagstaff, AZ; Medtronic, Minneapolis, MN; and Cook, Bloomington, IN). In the fenestration procedure, a tear in the dissection flap is created using intravascular ultrasound and fluoroscopic guidance, near the critical affected branch arteries. This is generally either near the level of the celiac trunk and just below the level of the renal arteries. A transjugular intrahepatic portosystemic shunt (TIPS) needle or other suitable curved needle is poised perpendicular to the flap and pushed through it, using a short, controlled jab (Figures 1 and 3). The hole in the flap is subsequently dilated with a 14-mm or similar angioplasty balloon, resulting in a transverse tear in the aorta, whose edges are drawn apart in a biconcave configuration by inherent longitudinal tension in the dissection flap (Figure 3D). Occasionally, intravascular ultrasound shows immediate relief of true lumen collapse. If collapse of the true lumen persists, a large-diameter self-expanding stent (such as 16 mm) is deployed in the aortic true lumen.
Endovascular Treatment of Aortic Dissection
Malperfusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
