Endovascular Thoracoabdominal Aortic Aneurysm Repair
GUILLERMO A. ESCOBAR
Presentation
A 65-year-old male presents to the emergency room with a 3-month history of progressive back pain that was unrelieved by oral pain medications and multiple visits to a chiropractor. He also states he has had progressive, painless dysphasia that was evaluated at an outside hospital with an upper endoscopy and was found to be negative for malignancy or hernia. He has lost approximately 10 kg of weight over the last 2 months and complains of significant overall deconditioning and weakness. His past medical history is notable for smoking 40 pack-years, hypertension, and coronary artery disease treated with the stenting 2 years ago. He has a previous open repair of an infrarenal abdominal aortic aneurysm 5 years ago. While currently unable to do so now, he walked on a treadmill 1 mile a day prior to this event.
Differential Diagnosis
Dysphasia can be characterized by either obstructive or functional diseases of the esophagus. With the combination of back pain and dysphasia in a 65-year-old male, malignancy should be considered first. However, a negative endoscopy increases the odds of an extrinsic esophageal compression or a functional disorder of the esophagus. Hiatal and diaphragmatic hernias may also present with dysphasia and intermittent back pain mostly associated with eating. Malignancies originating from the pulmonary or intrathoracic lymphatic systems can also encase the esophagus.
Vascular causes for dysphasia would include extrinsic compression from dysphagia lusoria or intrathoracic aneurysm disease. Dysphagia lusoria occurs when the right subclavian artery has an anomalous origin on the left side of the aortic arch, wraps behind the esophagus, and compresses it against the trachea. Aneurysms of the aortic arch, great vessels, or descending thoracic aorta may all compress the esophagus. These aneurysms may be primary, mycotic, or associated with a chronic dissection, and they can even erode into the esophagus leading to aortoesophageal fistulae.
Progressive back pain can be a primary disease of the musculoskeletal system or referred pain from a large variety of intrathoracic etiologies. Because extrinsic compression of the esophagus will generally lead to chronic dilatation, progressive back pain would not be expected unless there was an esophageal leak and mediastinitis. While acute aortic dissection could manifest with back pain, unless there was also an aneurysm, dysphasia would not be present as in this case.
A CTA would be the best step to distinguish the possible etiology of this patient. If negative, one would evaluate for esophageal dysmotility using a barium swallow. Because the barium would negate the visibility of the CT scan from scatter artifact, this should be done after the CTA if necessary (Fig. 1).
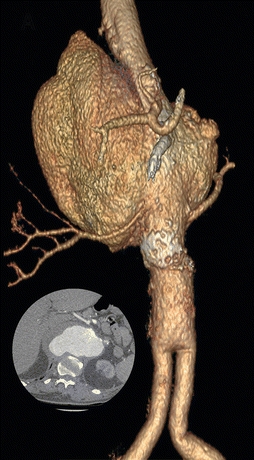
FIGURE 1 3D reconstruction of a large type IV TAAA. Inset is an axial cut demonstrating the largest portion of the TAAA. Note the aortobiiliac graft in the distal aorta.
CTA Report
A 13-cm fusiform thoracoabdominal aortic aneurysm involving the distal third of the thoracic aorta, that includes both mesenteric and renal arteries. The aneurysm ends just before an aortobiiliac bypass graft. There is no evidence of malignancy in the chest or abdomen. There is no evidence of inflammation in the mediastinum. The celiac artery appears occluded at its origin with a well-developed gastroduodenal artery. All three branches of the celiac are patent.
Discussion
Of all aneurysms of the thoracic aorta, the most common are ascending aortic aneurysms (40%), while thoracoabdominal aneurysms (TAAAs) only account for 10%. Almost 25% of patients with thoracic aortic aneurysms also have an abdominal aortic aneurysm. The shape of the aneurysm can help the clinician anticipate additional challenges. Most TAAAs are fusiform, and while penetrating ulcers and saccular aneurysms may be atherosclerotic, approximately 93% of mycotic aneurysms are saccular.
According to the Crawford classification of TAAAs, Crawford extent (type) IV aneurysms are limited to the aorta below the diaphragm and above the iliac bifurcation and are the most common (52%). Extent II TAAAs are the next most common (23%) and begin at the left subclavian artery down to the iliacs. Those beginning in the midthoracic aorta and extending through the mesenteric and renal vessels are “extent III” and account for 15%. Type I (9%) TAAAs are limited to the intrathoracic aorta from the left subclavian artery to the diaphragm.
Case Continued
His vital signs on presentation are blood pressure 180/90, heart rate 92, temperature 37.5, respiratory rate 18, and an oxygen saturation of 90% on room air. He is thin, and visibly uncomfortable due to his back pain. His chest is barrel shaped and clear to auscultation bilaterally. Abdominal exam reveals a scaphoid abdomen with a well-healed midline scar. There is a palpable pulsatile mass in the epigastrium. He has palpable pulses in all four extremities. His laboratory workup is notable for a creatinine of 1.3 mg/dL, blood urea nitrogen of 40 mg/dL, and a hemoglobin of 14 g/dL.
Discussion
For every increase of 1 cm above a 5-cm descending thoracic aorta, there is almost a doubling of the rupture risk. Within 2 years of diagnosis, a TAAA greater than 5.6 cm has a risk of death from rupture of approximately 25%. Generally, because patients with these aneurysms tend to have other significant comorbidities, a predicted survival greater than 25% at 2 to 4 years from other causes needs to be established prior to considering open repair. Crawford and DeNatale followed 94 patients who were not candidates for surgery, and 80% of those that ruptured had COPD.
This patient is relatively young but has become significantly deconditioned, malnourished, and dehydrated (elevated BUN and creatinine) from incapacitating back pain and dysphasia. His relatively low oxygen saturation suggests COPD in association to his smoking history. The very large size of his aneurysm and his progressive back pain are highly suggestive of imminent rupture, and a mycotic etiology must be considered. The combination of clinical findings is concerning for a poor outcome for traditional open surgery; however, conservative management is also very high risk for rupture and death.
Alternative options for managing this aneurysm would include endovascular approaches or hybrid (aortic debranching) techniques. The latter would still require an open abdominal exposure and bypasses to all four visceral vessels, in the setting of a previous abdominal aortic aneurysm repair. Avoiding any open procedure so as to limit further deconditioning makes endovascular a more attractive option. Four-vessel, totally endovascular management of a thoracoabdominal aneurysm is not available in the United States outside of investigational studies. Therefore, this only leaves physician-modified fenestration/branching of endografts or the use of parallel grafts (snorkels, chimneys, periscopes, and “sandwich” techniques) to maintain perfusion into the renal and mesenteric vessels, while excluding the thoracoabdominal aortic aneurysm.
Case Conclusion
A long discussion was had with the patient regarding his options, and appropriate institutional review board approval was obtained to perform an endovascular treatment for his thoracoabdominal aortic aneurysm. Access was obtained via cutdown of bilateral common femoral arteries, and a “preclose technique” was used to access his left axillary artery. The right lower extremity access was used to cannulate the left renal artery. This was aided by slowly injecting contrast while gently pulling back a cobra catheter on the aneurysm wall until the vessel was identified. The right renal artery was accessed via the left lower extremity, and the superior mesenteric artery was cannulated via the left axillary artery access. Rosen wires were placed inside of the visceral vessels, and subsequently, long sheaths were inserted into each. In a separate site on the right common femoral artery, a large sheath was placed inside of the abdominal aorta in order to deploy the thoracic stent graft. The aortic stent graft was advanced and put into position such that the distal portion would end approximately 5 mm before the bifurcation of his previously placed bifurcated Dacron graft. Fifteen-cm-long covered stent grafts (Gore Viabahn) were first deployed into the visceral vessels, and this was followed by placement of long angioplasty balloons into all three of the visceral vessels. These were insufflated while simultaneously deploying the thoracic aortic endograft (Gore cTAG). Using an aortic balloon, we expanded the aortic endograft while simultaneously insufflating the balloons in our visceral vessels. We determined that the overlap in the thoracic aorta was insufficient for an adequate seal between it and the superior mesenteric artery stent graft. Therefore, an additional aortic stent graft and covered stent graft was deployed into both the thoracic aorta and SMA, respectively, to extend the proximal seal zone. Simultaneous ballooning of the SMA and aortic grafts was again performed. Completion angiogram demonstrated no endoleak (Table1).



