Chapter 36 Endovascular Therapy for Aortic Dissection
Acute aortic dissection (AAD) is a precipitous event associated with a wide range of outcomes from uncomplicated to catastrophic. Current endovascular strategies are based on identifying features that portend increased risk of death or other poor outcome and applying interventional techniques to prevent the life-threatening complications of the dissection.1–3
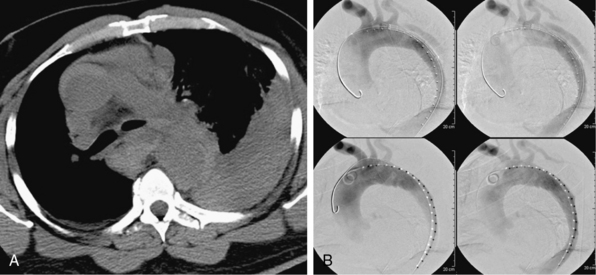
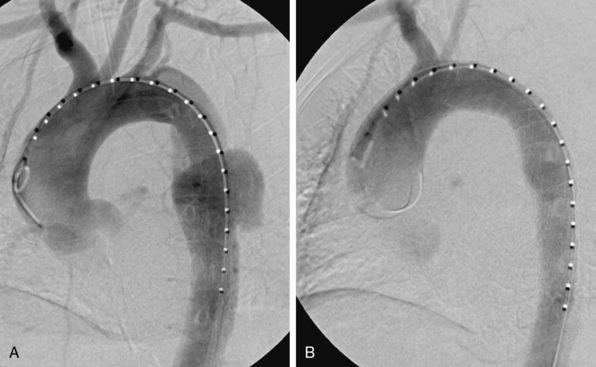
During the last 2 decades, there has been increasing interest in exploring endovascular procedures for management of aortic dissection.4–13 Initially, endovascular approaches focused on addressing branch vessel involvement and ischemic complications associated with the dissection process8,9 (Fig. 36-1). Subsequently, endovascular aortic stent grafts (initially developed to repair aortic aneurysms) were applied in type B aortic dissection to cover the primary entry tear of the dissection and promote thrombosis of the thoracic aortic false lumen4,5 (Fig. 36-2). These basic endovascular tactics are now routine in the contemporary armamentarium for treatment of aortic dissection and its myriad manifestations.
Branch Vessel Interventions
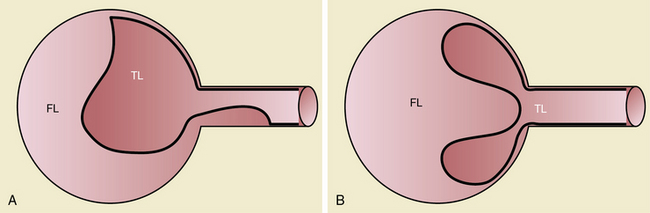
Branch vessel involvement accompanying aortic dissection is a well-recognized complication occurring in over 30% of cases.7,8,14 For appropriate intervention selection, the pathoanatomical concepts of static and dynamic branch involvement are crucial to selection of the endovascular option for reperfusion of an affected vascular bed.15–17 As the dissection process extends distally from the primary entry tear, the dissection septum may engage the ostia of branch vessels. If the aortic flap, which consists of the intima and portion of the media shorn away from the wall, engages a branch orifice as it extends, two pathophysiological situations referred to respectively as static and dynamic branch involvement may occur (Fig. 36-3).
Static Branch Involvement
One manifestation that may arise when the advancing dissection septum intersects an aortic branch is static branch vessel involvement (Fig. 36-4). In static involvement, the aortic dissection flap extends directly into the branch for a variable distance. In contrast to the geometry described earlier, orientation of the septal trajectory is such that the branch ostium is incompletely engaged by the edge of the dissection plane. Rather than being circumferentially shorn by the septum, there is only partial circumferential involvement of the branch by the dissection. The aortic flap extends into the branch, creating a false lumen within the artery. As a result, the individual branch has both a true and false lumen like the aorta.
In most cases, the preferred strategy involves increasing branch flow by decreasing the resistance to true lumen blood flow. This is performed by placing a stent in the true lumen of the branch through catheterization from the aortic true lumen. The stent is typically placed from beyond the end of the false lumen in the branch back to the aortic true lumen. A self-expanding nitinol stent is commonly employed because this distance is frequently greater than 2 cm and because there is a risk of squeezing any existing clot out of the false lumen with a balloon-expandable stent. These stents are sized to the total transarterial diameter of the branch and allowed to progressively expand on their own (post deployment) without supplemental balloon dilation. There are many successful reports of this approach in mesenteric, renal, and iliac arteries affected by no-reentry or static involvement.8,9,18,19
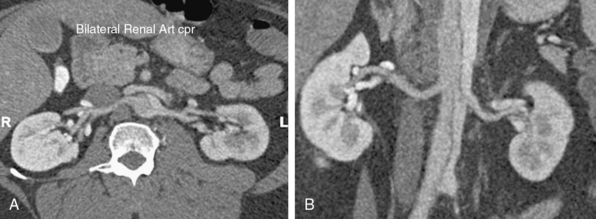
Occasionally, static branch vessel involvement with reentry anatomy and double-barrel flow may require endovascular intervention. The most common indication for stent placement in this setting occurs with involvement of a renal artery (Fig. 36-5). The kidney supplied by a dissected renal artery may be affected by the physical presence of a flap within the branch. The variable flow reduction caused by the flap, and resultant disrupted pattern of true and false lumen perfusion, may contribute to an exacerbation of hypertension. In cases where high blood pressure is sustained and recalcitrant to numerous intravenous (IV) medications, endovascular intervention may be warranted to restore a single lumen without flap. The approach to treatment involves placement of a balloon-expandable renal stent within the true lumen of the renal artery through the aortic true lumen. In most cases, this type of reentry involvement does not extend into the branch as far as the no-reentry extension. Thus, stents less than 2-cm long are typically implanted. This technique is well established at most centers that manage cases of aortic dissection frequently.
Dynamic Branch Involvement
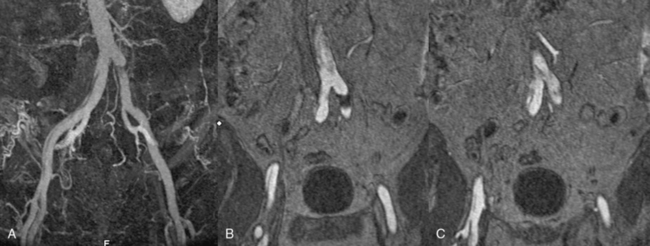
In addition to primary branch pathology that occurs as a complication of aortic dissection, another mechanism, dynamic branch vessel involvement, may be responsible for organ ischemia. Dynamic branch involvement is a phenomenon associated with obstruction to branch vessel flow by an aortic septum that has prolapsed over the branch ostia like a curtain. In contrast to static involvement, where the aortic flap extends directly into a branch, dynamic obstruction occurs as an aortic process exclusively without an associated branch lesion. Propagation of the aortic flap may create a circumferential cleavage of the aortic wall surrounding the branch ostium (Fig. 36-6). Factors associated with this event include the flap trajectory, the resultant orientation of the septal plane proximal to the branch, and the inclusion of the ostium by the cleaved flap as it extends past. In this situation, the dissection septum surrounds the branch ostium as it tears distally. The cleavage plane extends 1 to 2 mm into the branch, and then circumferentially reenters, creating a cylindrical tear, coring out a short segment of the intimal/medial lining of the most proximal aspect of the branch. The septum retracts into the aortic lumen with a fenestration corresponding to the branch orifice. This gives the flap a stencil-like appearance when viewed en face, with the number of holes related to the number of branch vessels involved by this phenomenon. When imaged in an axial plane, the affected artery appears to originate exclusively from the aortic false lumen. Closer inspection usually allows identification of a tear in the flap at the level or adjacent to the level of the branch. The flap often displays small projections angled from the edge of the tear, giving its outline on axial imaging an appearance similar to the contour of a metal rivet, the short-legged extensions corresponding to the amputated proximal lining of the branch.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree