Endovascular Revascularization for Renal Artery Occlusive Disease
Peter H. Lin
Ruth L. Bush
Alan B. Lumsden
Obstructive lesions of the renal artery can produce hypertension resulting in a condition known as renovascular hypertension, which is the most common form of hypertension amenable to therapeutic intervention. Renovascular hypertension is believed to affect 5% to 10% of all hypertensive patients in the United States. Patients with renovascular hypertension are at an increased risk for irreversible renal dysfunction if inadequate pharmacologic therapies are used to control the blood pressure. The majority of patients with renal artery obstructive disease have vascular lesions of either atherosclerotic disease or fibrodysplasia involving the renal arteries. The proximal portion of the renal artery represents the most common location for the development of atherosclerotic disease. It is well established that renal artery intervention, either by surgical or endovascular revascularization, provides an effective treatment for controlling renovascular hypertension as well as preserving renal function. The decision for intervention must encompass the full spectrum of clinical, anatomic, and physiologic considerations of the patient to yield the optimal benefit-risk balance.
Pathology of Renal Artery Stenosis
Approximately 80% of all renal artery occlusive lesions are caused by atherosclerosis, which typically occur near the renal artery ostia and are usually less than 1 cm in length (Fig. 42-1). Atherosclerotic lesions involving the renal artery origin account for more than 95% of reported cases of renovascular hypertension. Patients with this disease commonly present during the sixth decade of life. Men are affected twice as frequently as women. Moreover, they typically have other atherosclerotic disease involving the coronary, mesenteric, cerebrovascular, and peripheral arterial circulation. Atherosclerotic occlusive lesions involving the proximal renal artery typically occur as a spillover of diffuse aortic atherosclerosis, which is bilateral in more than two-thirds of patients. When a unilateral lesion is present, the disease process affects the right and left renal artery with similar frequency. Medial and intimal accumulations of fibrous plaque and cholesterol-laden foam cells are typical of the diseased renal artery wall. In more advanced disease, characteristics of complicated atherosclerotic plaques, such as hemorrhage, necrosis, calcification, and luminal thrombus, are commonly present in the renal artery wall.
The second most common cause of renal artery stenosis is fibromuscular dysplasia, which accounts for 20% of cases. Fibromuscular dysplasia of the renal artery represents a heterogeneous group of lesions that produces specific pathologic lesions in various regions of the vessel wall, including the intima, media, or adventitia. The most common variety consists of medial fibroplasia, in which thickened fibromuscular ridges alternate with attenuated media, producing the classic angiographic “string of beads” appearance (Fig. 42-2). The cause of medial fibroplasia remains unclear but appears to be associated with modification of arterial smooth muscle cells in response to estrogenic stimuli during the reproductive years, unusual traction forces on affected vessels, and mural ischemia from impairment of vasa vasorum blood flow. Fibromuscular hyperplasia usually affects the distal two-thirds of the main renal artery, and the right renal artery is affected more frequently than the left. The entity occurs most commonly in young, often multiparous women.
Other less common causes of renal artery stenosis include renal artery aneurysm (compressing the adjacent normal renal artery), arteriovenous malformations, neurofibromatosis, renal artery dissections, renal artery trauma, Takayasu arteritis, and renal arteriovenous fistula.
Clinical Features of Renal Artery Occlusive Disease
Renovascular hypertension is the most common sequelae of renal artery occlusive disease. Although the prevalence of renovascular hypertension is less than 5% in the general hypertensive population, this is one of the few treatable forms of hypertension. The prevalence of renovascular hypertension among patients with diastolic blood pressures greater than 100 mmHg is about 2%. It is even more frequent in patients who have severe diastolic hypertension, which can affect as many as 30% in those with a diastolic pressure over 125 mmHg.
Clinical features that suggest renovascular hypertension include:
Systolic and diastolic upper-abdominal bruits
Diastolic hypertension of greater than 115 mmHg
Rapid onset of hypertension after the age of 50
Sudden worsening of mild to moderate essential hypertension
Development of hypertension during childhood
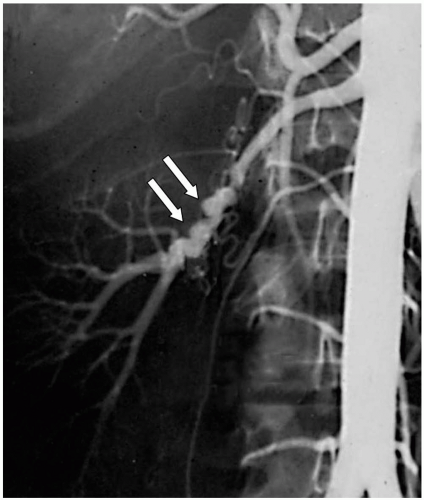 Figure 42-1. The typical “string of beads” appearance of renal artery fibromuscular dysplasia seen on an angiogram (arrows). |
Physical examination can provide an important diagnostic feature in detecting renovascular hypertension, particularly with the presence of an abdominal bruit located in the epigastrium or in either upper-abdominal quadrant. This findings is present in more than 75% of patients with renovascular hypertension, in contrast to less than 5% of those with essential hypertension. Hypertension resistant to pharmacologic therapy is also more likely to be associated with renovascular hypertension. In addition, those who develop renal function deterioration while receiving multiple antihypertensive drugs, particularly angiotensin-converting enzyme (ACE) inhibitors, may have underlying occlusive lesion involving the renal artery.
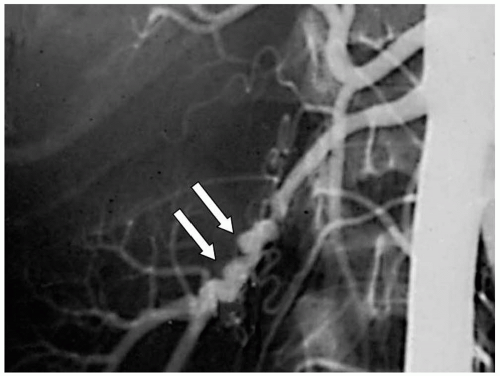 Figure 42-2. Occlusive disease of the renal artery typically involves the renal ostium, as a result of the aortic disease progression. |
All patients with significant hypertension, especially elevated diastolic blood pressure, must be considered as suspect for renovascular disease. Young adults with hypertension have a great deal to gain by avoiding lifelong treatment if renovascular hypertension is diagnosed and corrected. Appropriate diagnostic studies and intervention must be timely instituted to detect the possibility of renovascular hypertension in patients with primary hypertension who present for clinical evaluation.
Treatment Indications for Renal Artery Disease
The therapeutic goal in patients with renovascular disease is twofold. The first goal is to cure or improve high blood pressure, thereby preventing the long-term deleterious systemic sequelae of hypertension on target organ systems, such as the cerebral, coronary, pulmonary, and peripheral circulations. The second goal is to preserve and possibly improve the renal function.
Prior to 1990, the most common treatment modality in patients with renal artery occlusive disease was surgical revascularization, with either renal artery bypass grafting or renal artery endarterectomy. The advancement of endovascular therapy in the past decade has led to various minimally invasive treatment strategies, such as renal artery balloon angioplasty or stenting to control hypertension or to preserve renal function. The indications for endovascular treatment for renal artery occlusive disease include at least a 70% stenosis of one or both renal arteries and at least one of the following clinical criteria:
Inability to adequately control hypertension despite appropriate antihypertensive regimen
Chronic renal insufficiency related to bilateral renal artery occlusive disease or stenosis in a solitary functioning kidney
Dialysis-dependent renal failure in a patient with renal artery stenosis but without another definite cause of end-stage renal disease
Recurrent congestive heart failure or flash pulmonary edema not attributable to active coronary ischemia or other intrinsic cardiac disease
Endovascular Renal Artery Revascularization
Endovascular treatment of renal artery occlusive disease was first introduced in 1978 by Grüntzig, who successfully dilated a renal artery stenosis using a balloon catheter
technique. This technique requires passage of a guidewire under fluoroscopic control typically from a femoral artery approach to across the stenosis in the renal artery. A balloon dilating catheter is passed over the guidewire and positioned within the area of stenosis and inflated to produce a controlled disruption of the arterial wall. Alternatively, a balloon-mounted expandable stent can be used to primarily dilate the renal artery stenosis. Completion angiography is usually performed to assess the immediate results. The technical aspect of an endovascular renal artery revascularization is discussed below.
technique. This technique requires passage of a guidewire under fluoroscopic control typically from a femoral artery approach to across the stenosis in the renal artery. A balloon dilating catheter is passed over the guidewire and positioned within the area of stenosis and inflated to produce a controlled disruption of the arterial wall. Alternatively, a balloon-mounted expandable stent can be used to primarily dilate the renal artery stenosis. Completion angiography is usually performed to assess the immediate results. The technical aspect of an endovascular renal artery revascularization is discussed below.
Renal Artery Access and Guiding Sheath Placement
Access to the renal artery for endovascular intervention is typically performed via a femoral artery approach, although a brachial artery approach can be considered in the event of severe aortoiliac occlusive disease, aortoiliac aneurysm, or severe caudal renal artery angulation. Once an introducer sheath is placed in the femoral artery, an anteroposterior (AP) aortogram is obtained with a pigtail catheter placed in the suprarenal aorta to best visualize the left renal artery. In contrast, an aortogram with a 15° to 30° left anterior oblique (LAO) angulation is best to visualize the right renal artery. In patients with renal dysfunction, a selective renal catheterization can be performed without the initial aortogram. However, a diseased accessory or duplicating renal artery may be left undetected. Alternative noniodinated contrast agents, such as carbon dioxide and gadolinium, should be used in endovascular renal intervention in patients with renal dysfunction or allergic reactions.
Initial catheterization of the renal artery can be performed using a variety of selective angled catheters, which include the RDC, RC-2, Cobra-2, Simmons I, or SOS Omni catheter (Boston Scientific/Meditech, Natick, MA; Cook, Bloomington, IN; Medtronic, Santa Rosa, CA; Cordis, Warren, NJ; or Angiodynamics, Queensbury, NY). Once the renal artery is cannulated, systemic heparin (5,000 IU) is administered intravenously. A selective renal angiogram is then performed using a hand-injection technique with iso-osmolar contrast (Visipaque 270, Nycomed Amersham, Princeton, NJ). Once the diseased renal artery is identified, a 0.035″ or smaller profile 0.018″ to 0.014″ coronary guidewire is used to cross the stenotic lesion. Once the guidewire traverses the renal artery stenosis, the catheter is carefully advanced over the guidewire across the lesion. A vasodilator (e.g., glycerol trinitrite 150 µg) is administered in the renal artery through the catheter to minimize the possibility of renal artery spasm. If the renal artery is severely angulated as it arises from the aorta, a second, stiffer guidewire (Amplatz or Rosen Guidewire, Boston Scientific) may be exchanged through the catheter to facilitate the placement of a 45-cm 6-French renal guiding sheath (Pinnacle, Boston Scientific). It is important to maintain the distal wire position without movement in the tertiary renal branches during guiding sheath placement to reduce the possibility of parenchymal perforation. Once the guiding sheath, along with its tapered obturator, is advanced into the renal artery over the guidewire, the obturator is removed so the guiding sheath is positioned just proximal to the renal ostium. Selective renal angiogram is performed to ensure the proper positioning of the guiding sheath.
Renal Artery Balloon Angioplasty
With the image intensifier angled to maximize the visualization of the proximal renal artery, an angioplasty balloon is advanced over the guidewire through the guiding sheath and positioned across the renal artery stenosis. The balloon diameter should be chosen based on the vessel size of the adjacent normal renal artery segment. Various compliant angioplasty balloon catheters (CrossSail, Guidant, St. Paul, MN; or Gazelle, Boston Scientific) can be used for renal artery dilatation. We recommend choosing an angioplasty balloon less than 4 mm in diameter for the initial renal artery dilatation. The luminal diameter of the renal artery can be further assessed by measuring the known diameter of a fully inflated angioplasty balloon and comparing that to the renal artery dimension. Such a comparison may provide a reference guide to determining whether renal artery dilatation with a larger diameter angioplasty balloon is necessary.
Renal Artery Stent Placement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


