Endovascular Revascularization for Aortoiliac Occlusive Disease
Matthew J. Dougherty
Keith D. Calligaro
Diagnosis and management of aortoiliac occlusive disease (AIOD) continues to represent a significant portion of most vascular surgeons’ practices. The widespread acquisition of endovascular skills by vascular surgeons and the rapid evolution of endovascular technologies in recent years have led to a dramatic increase in the proportion of catheter-based interventions performed compared with traditional surgical procedures. This chapter reviews our approach to endovascular revascularization for AIOD.
Diagnostic Considerations
The majority of patients with aortoiliac occlusive disease present with claudication or limb-threatening ischemia. Severe ischemia is fairly straightforward from a diagnostic standpoint. The character of symptoms is most discriminatory, with complaints of pain or numbness at the distal lower extremity, usually the toes or forefoot. Pain is typically aggravated by elevation, worse at night, and relieved by dependency. Physical findings may include coolness, pallor, and dependent rubor. If accompanied by ischemic ulceration, lesions typically are at the acral aspect of the digits. The most useful initial diagnostic test is an arterial noninvasive evaluation, including segmental Doppler pressures and pulse volume recordings (PVRs). In patients with resting symptoms, there will be marked attenuation of PVR waveforms, usually accompanied by ankle-brachial index (ABI) below .40. We find the high thigh pressure and PVR to be very helpful in quantifying the contribution of aortoiliac disease in these patients who usually have multilevel occlusive disease. A significantly diminished high thigh pressure and PVR suggest that the inflow component of the disease is a major factor.
Patients with claudication symptoms can be more difficult to sort out than those with limb-threatening ischemia. There is significant overlap in symptomatology between AIOD and orthopedic conditions such as spinal stenosis. Both are common problems in older patients, and discriminating which pathology is primarily responsible for the patient’s symptoms can be challenging. Claudication symptoms should be highly predictable and reproducible at fixed distances. While thigh and buttock pain with walking are classic for AIOD, calf claudication is actually a more common presenting complaint. Typically walking on an uphill grade will be especially difficult with aortoiliac disease, as in addition to the calf muscles, quadriceps muscle perfusion is embarrassed. Patients with claudication secondary to AOID should report that standing in place relieves the discomfort, which is always in the muscle rather than the joints. After a brief rest, the claudicant should be able to walk a similar distance before reproducing symptoms. In contrast, spinal stenosis symptoms tend to be more variable, sometimes occurring even with standing, and they are frequently exacerbated by positional changes. There is often associated low back pain. Typically patients note that resting while standing does not relieve symptoms, but relief from weight bearing does. Nonetheless there is significant overlap of the symptoms of both conditions, and discriminating the contribution of vascular versus orthopedic disease can be difficult.
As with limb-threatening ischemia, the noninvasive vascular laboratory evaluation is critical. For claudicants, the critical components are the postexercise PVRs and Doppler pressures. After exercise, marked attenuation of PVR waveforms (usually to nearly flat-line) and ABIs should be observed with reproduction of symptoms. In our experience, failure to observe this indicates either a suboptimal exercise protocol, or, more often, an alternative cause for symptoms aside from arterial insufficiency.
Once the vascular specialist has determined that aortoiliac disease is indeed responsible for symptoms, color duplex arterial evaluation can be helpful. We feel strongly that the role of duplex, like arteriography, is to help define treatment options once the diagnosis of arterial insufficiency symptoms has been established by the aforementioned functional vascular laboratory studies. The major arteries can usually be visualized from the aorta to the trifurcation vessels. Occlusions can be defined with Bmode and color mapping, and stenosis graded based on peak systolic flow velocity (PSV) elevations. The latter are usually quantified based on the PSV ratio to adjacent patent segments, as has been standardized with graft duplex surveillance protocols.
We use duplex arterial mapping chiefly to help plan catheter-based interventions. This frequently allows us to forego complete diagnostic arteriography, and mobile C-arm digital arteriography is usually sufficient for these focused examinations. Nonetheless, good quality imaging is still critical, and when it is unclear that treatment of aortoiliac level disease will be used as initial therapy, we will generally perform complete diagnostic arteriography.
In general, two-plane images of the iliac arteries are obtained. If a stenotic lesion is of uncertain hemodynamic significance, a “pullback” pressure will be measured. A gradient of 15% of systolic pressure is considered significant. If there is no significant gradient at rest, exercise conditions are approximated by injecting 30 mg of papaverine into the iliac artery. The pullback gradient is remeasured, again defining a greater than 15% drop as significant.
Pathogenesis
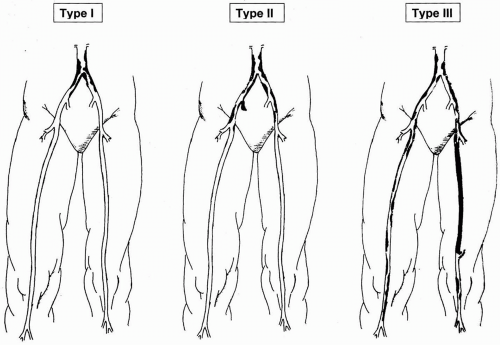
The majority of patients treated for occlusive disease in the aortoiliac segment have atherosclerotic plaque as the cause of stenotic or occlusive lesions. Atherosclerosis is a systemic process, with well-described risk factors including tobacco use, diabetes mellitus, hypertension, hyperlipidemia, and genetic predisposition. The aortoiliac segment is second only to the superficial femoral artery among peripheral vessels in frequency of involvement with hemodynamically significant plaque. Aortoiliac atherosclerosis is more prevalent among younger patients with occlusive disease. The distribution of plaque within the aortoiliac segments is classified in three types (Fig. 47-1).
Type 1 disease is confined to the infrarenal aorta and very proximal common iliac arteries. This pattern is more common in young smokers, and while there is a male predominance for AIOD overall, Type 1 pathology is more common in females. This pattern is found in only 5% to 10% of patients with AIOD. Type II disease is more common, with more diffuse involvement of the iliac arteries, particularly the external iliac artery. More common still is Type III disease, where in addition to the aortoiliac segments there is superficial femoral artery and infrapopliteal occlusive disease.
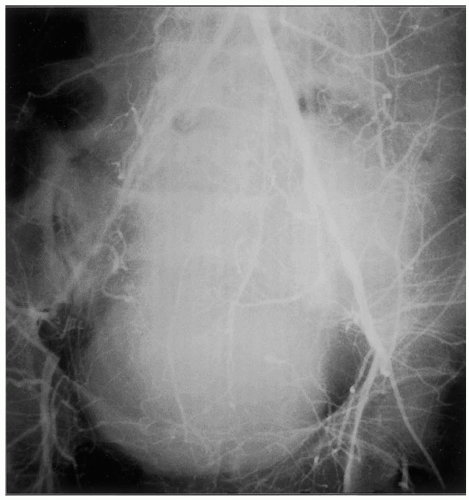
Aside from atherosclerosis, some less common arterial pathologies can involve the aortoiliac segment. Radiation arteritis is typically observed years after pelvic irradiation for gynecologic, genitourinary, or rectal cancers. While frequently accompanied by accelerated atherosclerosis, the lesions tend to be more fibrous in nature, involving relatively long arterial segments within the radiation field in a fairly uniform fashion (Fig. 47-2). Fibromuscular dysplasia has been described in the aortoiliac segment, and it tends to involve the external iliac arteries. Trauma, such as iatrogenic injuries with dissection or thrombosis related to catheterization injuries, is another occasional cause of AIOD. Vasculitis and congenital abnormalities can rarely involve the infrarenal aortoiliac segment. An uncommon lesion affecting the external iliac artery has been observed in avid cyclists, consisting of intimal fibrosis with smooth muscle hyperplasia, thought to be secondary to repetitive trauma.
Indications and Contraindications
The most common indications for intervention for AIOD are treatment of limb-threatening ischemia and failure of conservative treatment for lifestyle-limiting claudication. Another indication would be preservation of patency of an existing bypass graft distal to a stenotic lesion at the aortoiliac level. Last, some patients present with distal atheroembolization with plaque confined to
the aortoiliac segment, and treatment is aimed at preventing further embolic events.
the aortoiliac segment, and treatment is aimed at preventing further embolic events.
With limb-threatening ischemia, the most common clinical question is whether treating AIOD alone will be adequate to relieve symptoms of rest pain or accomplish healing of pedal breakdown. More often than not, tandem disease in the femoropopliteal segment exists in these patients (Type III disease), and treating AIOD alone, by either endovascular or open surgical measures, may or may not be sufficient. We find that comparing the high thigh pressure to the ankle pressure is helpful here. If there is a larger difference in systolic pressure from the brachial artery to the high thigh than from the high thigh to the ankle, chances are good that treating the AIOD alone will be sufficient (at least to relieve rest pain), as long as a good quality profunda femoris artery is patent with collaterals to the genicular region. While we do not hesitate to stage procedures when there is uncertainty about the extent of revascularization needed, if it appears likely that both endovascular treatment for AIOD and surgical infrainguinal revascularization will be necessary, we usually perform both as a combined procedure. This avoids the cost and inconvenience of multiple invasive procedures and does not appear to add significant morbidity to the open operative procedure.
For claudicants, indications for intervention are more subjective. We feel strongly that risk factor modification should be the first-line treatment. This includes complete cessation of tobacco use, aggressive therapy for hyperlipidemia, and to the extent that it can be accomplished, a regular exercise program consisting of a minimum of 30 minutes of walking daily. We offer a trial of cilostazol to all claudicants as well.
The appeal of the less invasive nature of catheter interventions has undoubtedly lowered the threshold for which some specialists and patients are willing to intervene. However, the small but real risk of major complications from endovascular therapy and the poor long-term results in patients with untreated risk factors mitigate against intervention in this setting.
For infrainguinal graft preservation, the role of endovascular treatment of AIOD is less well defined. However, grafts can occlude secondary to progression of inflow disease. The degree of inflow stenosis that can be tolerated without risk of thrombosis probably depends on the quality of the bypass conduit and outflow. In the presence of iliac stenosis, good caliber vein grafts to good outflow vessels are less likely to fail than a prosthetic bypass to a resistant outflow bed. If graft flow velocities significantly drop with the development of an iliac lesion, if the patient develops claudication, or if the high thigh PVR quality attenuates, the AIOD should be treated prophylactically for graft preservation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree