Endovenous laser ablation was initially performed in the operating room or radiology suite using conscious sedation or general anesthesia, but most procedures can now be safely performed under US guidance in the office setting under local anesthesia with or without sedation. The ablation procedure described for the GSV or SSV is similar to that for non-saphenous veins. Ligation of the saphenous vein near the deep venous junction, as advocated by some, is neither necessary nor desirable.
The patient is placed on an adjustable operating table with Trendelenburg capability. The course of the saphenous vein, from the saphenofemoral or saphenopopliteal junction to the insertion site, is mapped by US. An insertion site is chosen to maximize treatment length, minimize risk of thermal damage to perivenous structures, and ensure facile access. Most physicians use a site in the distal thigh or proximal calf for the great saphenous vein and the mid to distal calf for the small saphenous vein. Given extensive US-guided experience, some advocate treatment of the entire length of incompetent vein with little or no increased risk of paresthesia from thermal injury to perivenous nerves.
Access to the target vein is most often gained using an ultrasound-guided percutaneously placed needle followed by a guidewire, as in the Seldinger technique. Maneuvers such as placing the patient in a semierect position or applying 2% topical nitroglycerin ointment (Nitropaste) to the proposed insertion site before the sterile surgical prep can enhance successful venous cannulation by dilating the vein and preventing venospasm. It is sometimes appropriate to choose a primary access site and a higher, larger-diameter secondary (backup) access site in case access at the primary site is unsuccessful. As the practitioner’s US technical skills improve, even small-diameter saphenous veins can be successfully cannulated.
The first attempt at cannulation of the vein is the most likely to be successful, so the insertion site should be carefully chosen to make access as ergonomically feasible as possible. Just below the knee, the great saphenous vein is relatively anterior, and with the patient’s operative leg externally rotated, this site becomes more advantageous than in the distal or mid thigh.
After infiltration of local anesthetic at the insertion site, an introducer needle is inserted into the vein under US guidance. A microinsertion set can be used to gain access for the sheath that will accommodate a 600-μm laser fiber. Using the Seldinger technique and guidewire, the sheath is advanced into the vein over the guidewire until it is identified by US to be 3 to 4 cm below the saphenofemoral junction or just inferior to the deep penetration of the SSV where it joins the deep system. Alternatively, the bare-tipped fiber may be advanced directly through the access needle or the short sheath in the micropuncture set and carefully guided to the same position.
Occasionally, passage of the fiber is impeded by vein tortuosity. Straightening the leg or gently guiding the fiber by external compression and manipulation of the thigh usually allows successful advancement. Segmental stenosis from previous sclerotherapy or thrombophlebitis also impedes advancement of the fiber or guidewire. In this case, or if the vein is so tortuous as to not allow passage, a second cannulation allows treatment of first the proximal and then the distal segments of the saphenous vein.
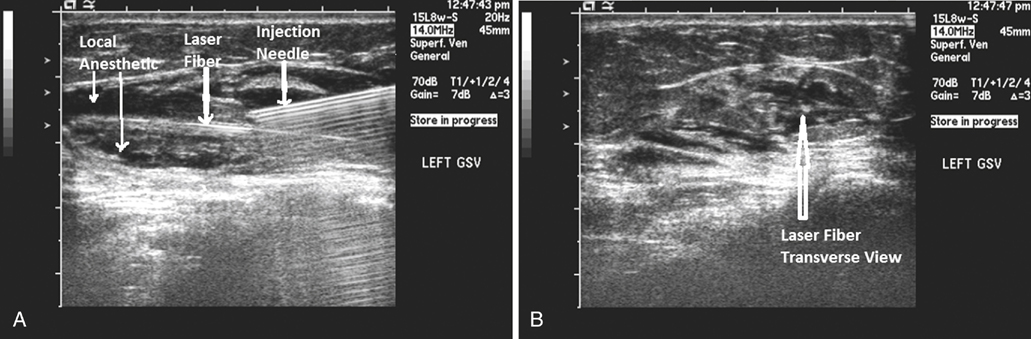
US-guided dilute anesthesia (0.05%–0.25% lidocaine with epinephrine and bicarbonate) is then injected into the saphenous compartment (Figure 1), completely surrounding the target vein to ensure an adequate anesthetic effect. The tumescent local anesthetic causes the vein to become contracted for better thermal effect and to protect the perivenous structures from thermal damage. It is always necessary to clearly identify important anatomic landmarks (Figure 2) near the deep venous junction before injecting the local anesthetic, which will obscure these landmarks, severely limiting visualization of the correct position of the laser tip. The tumescence of the well placed anesthetic provides compression of the vein, which in turn limits the volume of blood within the vein which enhances the effect of the laser energy on the vein wall.
Only gold members can continue reading.
Log In or
Register to continue
Related
![]()



