and Nicholas M. Southard1
(1)
Division of Vascular Surgery, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA
7.1 Introduction
7.2.1 Basic Techniques
7.2.2 Systems Issues
7.2.3 Radiation Safety
7.3.1 Head and Neck
7.3.2 Thoracic Aortic Injury
7.4 Conclusion
Abstract
The last two decades have seen a dramatic shift in the management of vascular disease from traditional open surgery to complex endovascular techniques. While this trend is quite well documented in the treatment of aneurysms of the abdominal and thoracic aorta, these changes have likewise taken place in management of vascular occlusive disease, and the technical aspects of these procedures can be applied to selected cases of vascular trauma. While endovascular management of blunt thoracic aortic injury has been increasingly reported with positive results, few data exist regarding safety and efficacy of endovascular management of injury to other vascular distributions. Selecting appropriate injuries for endovascular management is a particular challenge. There is a general perception that patients with acute arterial injuries – particularly hemodynamically unstable patients with ongoing hemorrhage – are inappropriate for endovascular management, despite the large volume of data showing improved outcomes in unstable patients with ruptured aortic aneurysms managed with a primary endovascular approach.
7.1 Introduction
The last two decades have seen a dramatic shift in the management of vascular disease from traditional open surgery to complex endovascular techniques. While this trend is quite well documented in the treatment of aneurysms of the abdominal and thoracic aorta, these changes have likewise taken place in management of vascular occlusive disease, and the technical aspects of these procedures can be applied to selected cases of vascular trauma. While endovascular management of blunt thoracic aortic injury has been increasingly reported with positive results, few data exist regarding safety and efficacy of endovascular management of injury to other vascular distributions.
Selecting appropriate injuries for endovascular management is a particular challenge. There is a general perception that patients with acute arterial injuries – particularly hemodynamically unstable patients with ongoing hemorrhage – are inappropriate for endovascular management, despite the large volume of data showing improved outcomes in unstable patients with ruptured aortic aneurysms managed with a primary endovascular approach [1]. An increasing percentage of both blunt and penetrating vascular injuries in the USA are being managed by vascular surgeons with endovascular techniques, and it is of significant importance that general surgeons and trauma surgeons have an understanding of the situations in which endovascular therapy may be a viable and readily applied alternative to major open vascular reconstruction in severely injured patients [1, 2].
7.2 General Considerations
7.2.1 Basic Techniques
In all types of endovascular intervention, the basic concepts of catheter-directed treatment of a vascular lesion include access to the vascular tree at a remote site, crossing the offending lesion with a wire, and delivering a device that will allow correction of the lesion. The vast majority of these interventions are performed through an arterial access sheath (French size noted by the inner diameter of the device), with a steerable access catheter (French size noted by the outer diameter of the device) coaxially with a wire being guided to the appropriate area under direct fluoroscopic guidance. If the affected vascular territory is not at risk for ischemia, lesions can be treated simply by vascular occlusion (i.e., coil embolization of pelvic arterial bleeding, microparticle embolization of bleeding tumors). Major arterial vascular trauma is unique in that injuries must generally be repaired in such a way as to allow continued arterial flow beyond the injured vessel to avoid the consequences of ischemia. In these cases treatment can be considered with covered stent grafts.
In vascular territories where ischemia can be tolerated, embolization of bleeding vessels can be considered. Well-documented areas where these techniques are useful are in hemorrhage from splenic and hepatic injuries and pelvic trauma with arterial bleeding, as documented elsewhere in this text. Other areas where embolization can be quite useful are hemorrhage from external carotid branch injuries, intercostal artery bleeding, and for injuries to branch arteries of the extremities where collateral flow will allow distal perfusion in the absence of the injured target vessel. Embolization can be carried out with endovascular coils or vascular plugs for large vessels. For smaller vessels or for diffuse bleeding from injured surfaces, microembolization can be carried out with smaller particles such as polyvinyl alcohol (PVA) or N-butyl cyanoacrylate (NBCA) glue. These techniques require specialized imaging and catheter techniques and should be available on short notice at those trauma centers specializing in the endovascular treatment of traumatic vascular injuries.
Large vessel injuries (arterial and venous) can often be repaired with the deployment of covered stents. These are available in several varieties and sizes (both lengths and diameters) from multiple manufacturers and may be either self-expanding or balloon expandable. Exclusion of vascular injuries to prevent continued hemorrhage is accomplished by the deployment of a covered stent or an endograft within the lumen of a blood vessel. Ideally, deployment of these devices requires proximal and distal landing zones of 2 cm of normal artery and vein to ensure an adequate seal to prevent a type I endoleak. Furthermore, precise covered stent or endograft placement will be necessary so as not to cover important branches or bifurcations which may result in the distal ischemia or embolization.
Venous injuries are generally treated with similar principles, although the vast majority can be treated with simple occlusion rather than complex revascularization procedures. The majority of this chapter will focus on management of arterial injuries.
7.2.2 Systems Issues
Several publications have addressed the evolving role of endovascular management of vascular trauma. A large review of the National Trauma Data Bank (NTDB) found that from 2002 to 2008, the percentage of blunt vascular injuries managed by endovascular means increased from just over 1 % to over 17 % [1]. The percentage of penetrating vascular injuries managed using endovascular techniques during that time was relatively stable but did demonstrate a statistically significant increase from 2.6 to 3.2 %. When this paper was presented at the 2011 meeting of the American Association for Surgery of Trauma (AAST), the ensuing discussions centered to a large extent on who was performing the procedures and which particular procedures should be performed. As pointed out by Kalish, it is of paramount importance for the trauma surgeon and endovascular specialist to maintain good communication regarding the role of endovascular management for these severely injured patients, as some injuries may be more expeditiously managed by open surgery [3].
Of paramount importance to the rapid repair of vascular injuries is the ability to quickly convert between open and endovascular surgery. Traditionally, endovascular procedures have been performed in imaging suites remote from the operating room, with poor lighting and limited room to set up an open surgical case. Hybrid endovascular suites in the operating room environment help obviate these issues, and provided both vascular and trauma surgeons the ability to perform complex hybrid endovascular procedures without excessive delay (Fig. 7.1). These hybrid suites are particularly important in cases where it is important to determine location and extend of major vascular injuries in order to expeditiously deploy large covered endografts to stabilize the patient and prevent further bleeding. By having the ability to move from an endovascular to open surgical approach and vice versa, the surgeon afforded the flexibility to provide to gain arterial or venous access and deliver the best of both vascular and trauma surgical care in a single operating suite. Additionally, performing these cases in a hybrid operating room, as opposed to an interventional radiology suite or cardiology catheterization laboratory, provides an extra layer of comfort for trauma surgeons, vascular surgeons, and anesthesiologists as a result that any and all necessary instruments, medications, and personnel support are present and immediately available the operating team.
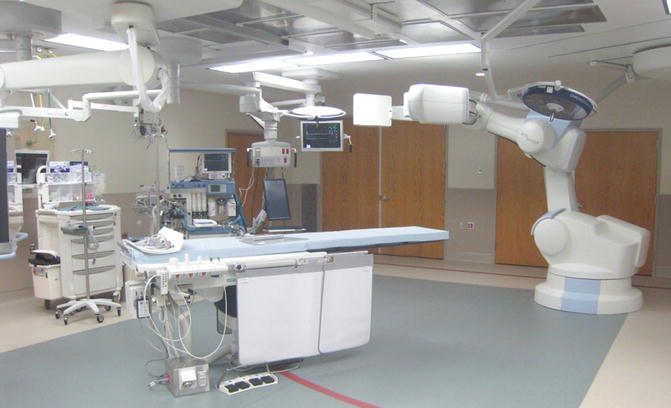

Fig. 7.1
Hybrid endovascular suite in main operating room. The floating table is fully capable of accommodating mounted retraction systems and has all movement capabilities (i.e., airplane, Trendelenburg) as standard operating room tables
7.2.3 Radiation Safety
Critically ill patients are exposed to numerous safety hazards [4]. The introduction of endovascular techniques to the care of trauma patients of necessity exposes patients and the interventional operating team to the effects of ionizing radiation, which can accumulate quickly in patients with multiple injuries which necessitates the need for multiple procedures and prolonged operative procedure times. Dose-dependent effects of ionizing radiation exposure include hypothyroidism, cataract formation, alopecia, and hematologic and solid organ malignancies [4]. All members of the interventional or operative team participating in endovascular procedures should wear wraparound lead aprons, thyroid shields, and lead-lined radiation protective glasses or goggles. It is underappreciated that the majority of radiation exposure during endovascular cases comes from beam scatter from the patient, not from the imaging device itself, and patterns of exposure can be unpredictable. Real-time monitoring of radiation dose exposure is important and each member should wear two radiation monitoring badges. One badge should be worn outside the neck collar at the level of the thyroid and the second badge should be worn inside the lead apron at waist level. Of particular importance in these procedures is minimizing exposure by proper use of collimation of the x-ray beam, intermittent rather than continuous fluoroscopy use, and use of last image hold techniques [5]. Additionally, it is important to keep the image detector of the C-arm as close to the patient as possible to minimize the scattering of the x-ray beam, which is the most common source of radiation exposure in these procedures.
7.3 Specific Management Principles
7.3.1 Head and Neck
Endovascular management of carotid and vertebral artery trauma has been documented for several years. One of the earliest reports of this examined numerous methods to treat traumatic carotid and vertebral lesions with embolization therapy, including the use of detachable balloons, liquid tissue adhesives, microcoils, and pieces of silk suture [6]. A large variety of lesions were treated, including carotid-cavernous fistulas, dural fistulas, and direct penetrating arteriovenous fistulas. Five out of 234 patients experienced strokes. The number of patients requiring open surgical therapy was not reported. Since that time, numerous case reports and series have documented techniques of management of carotid and vertebral artery injuries, all in a selective fashion. Herrera reported 36 cases of penetrating cervical carotid trauma treated with endovascular management over a 12-year period with successful repair in 34 cases [7]. No randomized trials have been reported, and long-term results are not available. Endovascular stent grafts have also been used for the treatment of traumatic jugular-carotid fistulas, to repair true and false aneurysms of the carotid arteries and to repair iatrogenic carotid injuries [8–10].
The distal internal carotid artery is an ideal location for application of endovascular management due to its difficult surgical access. Figure 7.2 is the initial angiogram of patient that presented to our institution with hemorrhage from the distal internal carotid artery as the result of a transcervical gunshot wound. The patient was brought to the hybrid operating room for an initial attempt at endovascular exclusion, with left neck exploration planned if unable to cross the lesion with a wire. The lesion was successfully excluded with a covered endograft (Fig. 7.3), with a total procedure time from femoral arterial puncture to lesion exclusion of 22 min. The patient did not require neck exploration; he did have a tracheostomy post-procedurally for airway management and was discharged home on postoperative day #19.
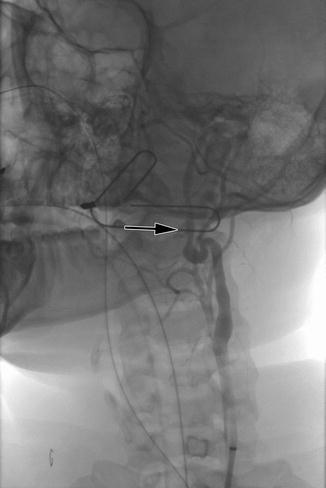
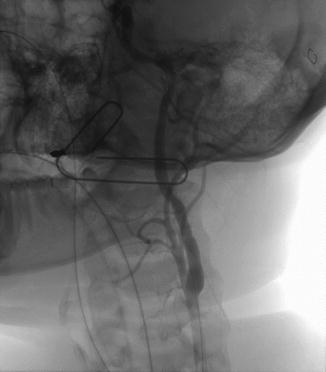

Fig. 7.2
Penetrating injury to the distal left internal carotid artery with false aneurysm formation (arrow)

Fig. 7.3
Successful exclusion of internal carotid penetrating injury with Viabahn (W.L. Gore, Flagstaff, AZ) covered stent
Blunt carotid injury is a potentially devastating condition that is notoriously difficult to diagnose, and the management of which remains controversial [11]. Patients with internal carotid dissection that either becomes symptomatic (transient ischemic attack [TIA], stroke, amaurosis) or worsens during surveillance are candidates for endovascular stenting. These patients are required to take antiplatelet agents (clopidogrel and aspirin) both pre- and post-procedurally, and this may be impractical in the setting of concomitant major trauma to other organ systems. Likewise, the natural history of stents in the internal carotid arteries of young patients is not clearly defined. When carotid stenting is required due to symptomatic dissections, or worsening dissection during surveillance, it should be done with embolic protection and with pre-procedural administration of antiplatelet medications. Although the use of embolic protection devices in these patients is controversial, they should be used when possible until more data are available for review [11].
7.3.2 Thoracic Aortic Injury
Blunt injury to the thoracic aorta is a highly lethal injury resulting in approximately 8,000 deaths in the United States annually [12, 13]. It is the second most common cause of trauma-related death, behind intracranial hemorrhage. Most patients who sustain this type of injury die at the scene with less than 25 % surviving long enough to be evaluated at a hospital [14]. Of those who present to the hospital with blunt aortic injury (BAI), up to 50 % die within 24 h [15].
Traumatic aortic injuries range from small defects in the intimal layer to complete transection of the artery, typically just beyond the origin of the left subclavian artery (LSA). Several classification schemes have been proposed based on radiologic imaging in an attempt to grade the various types of aortic defects according to severity of injury, which can be used to guide therapy. The Society of Vascular Surgery in their 2011 practice guidelines endorsed the classification scheme published by Azizzadeh [16]. In this scheme there are four grades of aortic injury, ranging in severity from grade I (intimal tear) to grade IV (free rupture) (Fig. 7.4).
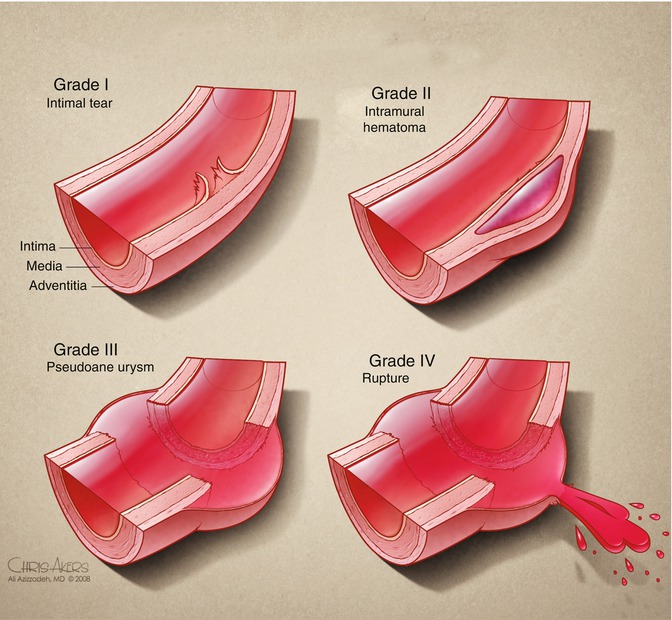

Fig. 7.4
Classification of traumatic aortic injury (From Azizzadeh 2009, used with permission)
Prior to the endovascular era, a majority of aortic injuries were repaired via a conventional surgical approach consisting of a left thoracotomy with or without cardiopulmonary bypass. Open repair for traumatic aortic injuries historically has been associated with a mortality rate approaching 30 % and a paraplegia rate of 16 % [17, 18]. The first thoracic endovascular aortic repair (TEVAR) was described in 1997 and, since then, has become the preferred technique to repair BAI [19]. TEVAR has proven to be a durable repair option for these critically ill patients and eliminates the morbidity associated with cross clamping of the aorta and the need for cardiopulmonary bypass required for an open repair. Although better tolerated by patients, TEVAR still carries a risk of spinal cord ischemia, stroke, and other complications that are associated with open repair.
There have not been randomized trials comparing open versus endovascular repair of traumatic aortic injury, but there have been several single institution reports as well as meta-analyses of the literature comparing the two procedures. In 2012, Risenman and colleagues reported their institutional experience over a 20-year period comparing 60 open repairs to 26 endovascular repairs [20]. They concluded that patients undergoing endovascular repair had a significantly lower intraoperative mortality rate (0 % vs. 18 %) and overall hospital mortality rate (12 % vs. 37 %) compared to open surgical repair [20]. Rousseau and colleagues reported their 22-year experience which revealed mortality and paraplegia rates of 21 and 7 %, respectively, in 28 patients undergoing open repair versus 0 % mortality and paraplegia rate in 29 patients undergoing endovascular repair of BAI [21]. Ott and colleagues reported an 11-year retrospective study comparing 12 open procedures to 6 endovascular procedures [18]. Mortality and paraplegia rates were 17 and 16 %, respectively, for the open group versus 0 % mortality and paraplegia rate for the endovascular group. In a recent meta-analysis, Tang et al. reviewed 33 published articles including 699 procedures (TEVAR – 370; open – 329) which showed a significantly lower rate of mortality (7.6 % vs. 15.2 %), paraplegia (0 % vs. 5.6 %), and stroke (0.85 % vs. 5.3 %) in patients who underwent TEVAR compared to open repair [22]. Collectively, outcomes from these reports and others have provided compelling data which has led to endovascular repair of traumatic aortic injury becoming the mainstay of therapy.
Traumatic aortic injuries should be suspected in trauma patients who present with mechanism of injury consistent with rapid deceleration. Historically, aortic injuries were suggested by abnormal chest x-rays and confirmed with arch aortography; however, with the improvements in high-resolution computed tomography scanners, they have been replaced by CTA as the preferred method of diagnosis. In addition, CTA imaging is instrumental in procedural planning.
Once an aortic injury has been confirmed, strict blood pressure control should be obtained. A goal systolic pressure between 100 and 120 mmHg and a heart rate of 60–80 is ideal. Multiple studies have shown that aggressive blood pressure and heart rate control is associated with improved outcomes in those suffering BAI [21, 23, 24]. In a review by Hemmila, it was shown that antihypertensive therapy started at the time of aortic injury diagnosis reduced the risk of aortic rupture to less than 1.5 % in patients whose aortic repair was delayed due to other life-threatening injuries taking precedence [25].
Approximately 10 % of patients with traumatic aortic injuries have grade I aortic injuries also referred to as minimal aortic injury [26]. These injuries can be managed with observation with the expectation that these injuries will heal spontaneously. These patients do however require serial imaging to monitor for injury progression. Grade II through IV aortic injuries should be repaired urgently within 24 h of diagnosis unless there are other more life-threatening cranial or intra-abdominal injuries which require more emergent intervention. In these patients, aortic repair can be delayed but should be performed prior to discharge [12, 27]. Hemmila reported that delay in surgical repair for traumatic aortic injury beyond 16 h was not associated with an increased risk of mortality [25].
Since there is a potential risk of paralysis associated with the repair of the thoracic aorta, some have advocated the placement of spinal drains prior to intervention. The use of spinal drainage for the management of spinal cord ischemia during TEVAR for aneurysmal disease has been routine; however, no data exist on the prophylactic use for traumatic injuries. Spinal cord ischemia following TEVAR occurs in only 3 % of patients due to the limited coverage of the thoracic aorta [28]. Because of the low risk of ischemia and the risk of epidural hematoma during placement in coagulopathic trauma patients, the Society for Vascular Surgery in their most recent consensus guidelines has recommended against the routine placement of spinal drains and stated that they should only be placed if symptoms of spinal cord ischemia develop [27].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


