Endovascular Composite Sequential Bypass Using Covered SFA Graft and Vein Bypass for Distal Reconstruction
Mark Patterson
Introduction
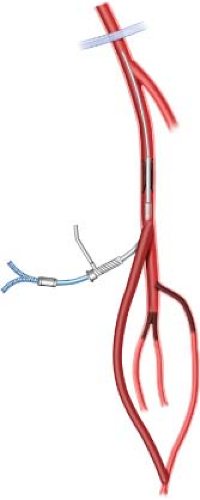
Lower extremity bypass remains the most durable therapy for critical limb ischemia. Classical teaching mandates using autologous conduit (greater saphenous vein) to bypass all occlusive lesions between a suitable inflow and recipient outflow artery. Occasionally, circumstances dictate modification of such practice to afford patients maximum opportunity for limb salvage. Prior to application of endovascular therapy in infrainguinal vascular beds, patients with limited autologous conduit and suitable anatomy underwent composite sequential bypass with inflow provided via prosthetic bypass to the popliteal level and outflow established via autologous bypass to a tibial or pedal artery. As experience with catheter-based revascularization has expanded, endovascular techniques to augment inflow via treatment of hemodynamically relevant superficial femoral artery (SFA) stenoses to allow distal vein bypass with alternate inflow sites and shorter autologous conduits has evolved. This approach has likewise proved a viable option and in my practice it is termed as “catheter-assisted composite sequential grafting” (CACG; Fig. 6.1).
Indications for lower extremity revascularization include lifestyle-limiting claudication and critical limb ischemia. Candidates for CACG almost exclusively exhibit critical limb ischemia typically presenting with nonhealing ulcerations or gangrene. Ischemic rest pain involving the fore-foot or toes without tissue loss may also be present. Anatomy
suitable for CACG involves presence of SFA stenosis or occlusive lesions, a preserved segment of above or below knee popliteal artery with concomitant tibioperoneal disease. Complete revascularization requires correction of the SFA abnormality to allow sufficient inflow to support a distal bypass originating at the popliteal level with tibial or pedal arteries used for outflow. Most often CACG is performed for diabetic patients with critical limb ischemia and limited autologous vein.
suitable for CACG involves presence of SFA stenosis or occlusive lesions, a preserved segment of above or below knee popliteal artery with concomitant tibioperoneal disease. Complete revascularization requires correction of the SFA abnormality to allow sufficient inflow to support a distal bypass originating at the popliteal level with tibial or pedal arteries used for outflow. Most often CACG is performed for diabetic patients with critical limb ischemia and limited autologous vein.
Efficient lower extremity revascularization relies upon creation and adherence to a thoroughly established, patient-specific surgical plan. Proper strategy requires delineation of arterial anatomy, autologous conduit availability, patient physiologic condition, surgeon-related technical proficiency, and facility inventory. As each component is considered and defined, the likelihood for successful revascularization is significantly enhanced.
Arterial Anatomy
Assessment of arterial anatomy begins in the outpatient setting during physical examination and review of noninvasive vascular laboratory data. The combination of physical examination, segmental limb pressures, with pulse volume recording waveform analysis allows assessment of groin-level inflow and provokes suspicion of distal arterial impairment. Most patients possess visible limb-threatening lesions and the need for diagnostic imaging is evident.
Definitive arterial anatomy assessment is most often achieved by intra-arterial digital subtraction angiography (DSA). Imaging of the entire arterial system from the renal arteries to the foot delineates all levels of arterial disease. Computed tomography angiography (CTA) has become popular and may serve as a planning tool, but rarely supplants the need for definitive DSA. If preferred, duplex ultrasound examination also provides an option for initial imaging. Duplex imaging delineates segmental infrainguinal lesions and avoids potentially harmful contrast exposure. It is pertinent to note that for duplex imaging to be maximally effective, a requisite level of skill is required of technicians performing the examination as well as among physicians interpreting the examination to allow accurate clinical decision-making. It is my practice to use CTA in
circumstances where noninvasive laboratory or physical examination findings suggest proximal aortoiliac lesions. DSA is used initially when groin-level inflow is appropriate and the primary concern is for more distal occlusive lesions.
circumstances where noninvasive laboratory or physical examination findings suggest proximal aortoiliac lesions. DSA is used initially when groin-level inflow is appropriate and the primary concern is for more distal occlusive lesions.
Table 6.1 TASC Classification of Femoral Popliteal Lesions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Properly executed angiography confirms suitable inflow and outflow targets as well as SFA lesions amenable to endovascular therapy. Iliac stenoses are addressed at the time of diagnostic DSA, while SFA lesions are treated selectively based upon TASC II classification (Table 6.1). TASC A or B lesions when identified are typically treated during the hybrid revascularization, particularly when the lesions consist primarily of stenoses. TASC C lesions consisting of long segment near occlusive lesions are usually reserved for the surgical revascularization as well. TASC C lesions with intermittent occlusions are approached during the index diagnostic angiogram. Similarly TASC D SFA lesions felt to be amenable to endovascular therapy are approached separate from the hybrid bypass procedure. This approach prevents the occasional misadventure with crossing occlusions from derailing the momentum of a hybrid surgical procedure. If recanalization of the occlusion is unsuccessful, alternate surgical plans are formulated.
Viabahn (W.L. Gore, Flagstaff, AZ) endoprostheses are typically used for SFA intervention. Other covered stent options include Fluency Plus (Bard, Tempe, AZ) or Wallgraft (Boston Scientific, Natick, MA). It is my opinion that covered devices most closely duplicate intraluminal flow characteristics associated with prosthetic graft revascularization and when sized and deployed properly, reduce intraoperative dissection and vessel injury.
Autologous Conduit
Careful history and physical examination allow preliminary assessment of available autologous conduit. Not infrequently patients have undergone greater saphenous vein harvest for coronary artery bypass or prior lower extremity bypass. I routinely evaluate all superficial lower extremity veins with B-mode ultrasound vein mapping. Ultrasound mapping allows assessment of conduit quality by delineating length of conduit available, vein diameter, patency, vein wall inflammation, or presence of varicosities. Circumstances in which lower extremity vein is limited, upper extremity veins are evaluated. As most of the necessary revascularizations require at most, sufficient conduit to reach from the above or below knee popliteal artery to the foot, the length of conduit required will vary and consistently be less than needed should SFA intervention be unsuccessful.
Patient Physiologic Assessment
Patients with critical limb ischemia harbor numerous medical conditions which require management and optimization during surgical planning. The most common associated conditions include coronary artery disease, hypertension, diabetes mellitus, hyperlipidemia,
and tobacco use. Communication and involvement of primary care physicians is helpful to assure optimization of blood pressure, glycemic control, and statin administration. Controversy persists as to the most appropriate cardiac screening prior to lower extremity bypass. Due to the severity of arterial ischemia, delay of lower extremity intervention is not typically an option to allow for cardiac intervention with exception of symptomatic coronary disease.
and tobacco use. Communication and involvement of primary care physicians is helpful to assure optimization of blood pressure, glycemic control, and statin administration. Controversy persists as to the most appropriate cardiac screening prior to lower extremity bypass. Due to the severity of arterial ischemia, delay of lower extremity intervention is not typically an option to allow for cardiac intervention with exception of symptomatic coronary disease.
Surgeon-related Technical Consideration
Incorporation of catheter-based intervention during open surgery has become more common as surgeon experience with endovascular procedures has expanded. Successful and efficient completion of hybrid procedures requires experience, insight, judgment, confidence, and technical proficiency to prevent either component of the revascularization from compromising anticipated success. Careful planning as to the most efficient sequence for each modality (endovascular and open revascularization) during the procedure followed by a detailed, start to finish, “conceptual or imaginary practice run” of the anticipated procedure is beneficial. To successfully execute both the endovascular and open surgical components of a hybrid procedure, the surgeon must be familiar with his or her endovascular inventory, various diameter platforms (0.035, 0.018, 0.014), over the wire and monorail techniques, as well as ancillary options for crossing occlusive lesions. The ability to troubleshoot, redirect, and avoid critical errors cannot be overstated. CACG procedures may be performed either in a hybrid operating suite with fixed imaging equipment, or in a standard operating room with a mobile C-arm system equipped with DSA capability.
Upon completion of preoperative assessment and development of a surgical plan, the decision to proceed with hybrid surgical intervention is confirmed. As noted previously, the key to success and minimal patient insult relies upon efficiency and simplicity. My approach with hybrid revascularizations is intended to minimize operative duration, maintain surgical momentum, and avoid duplication of effort.