Figure 29.1
Classical TBNA of a lymphnode station 7. The needle pierce the bronchial wall

Figure 29.2
THe EBUS-TBNA scope from OLympus
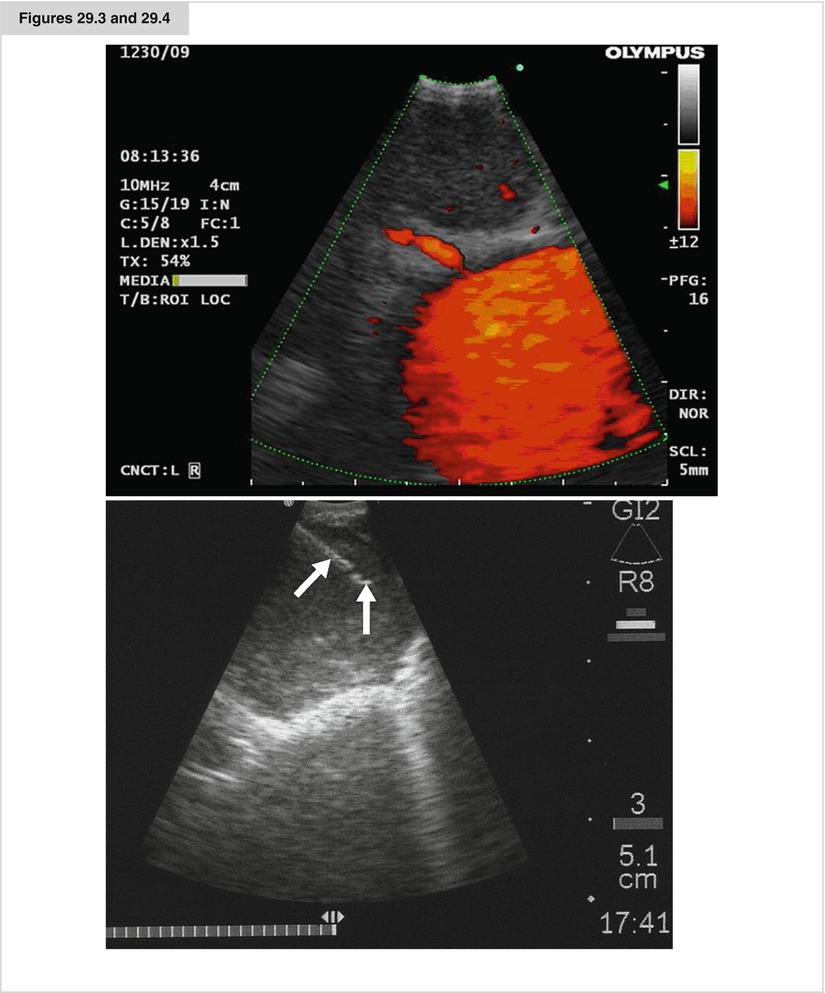
Figures 29.3 and 29.4
An EBUS image from an enlarged lymphnode. in orange the flow in the pulmonary artery (doppler-flow). An EBUS-TBNA of an enlarged lymph node. The needle is mark by arrows
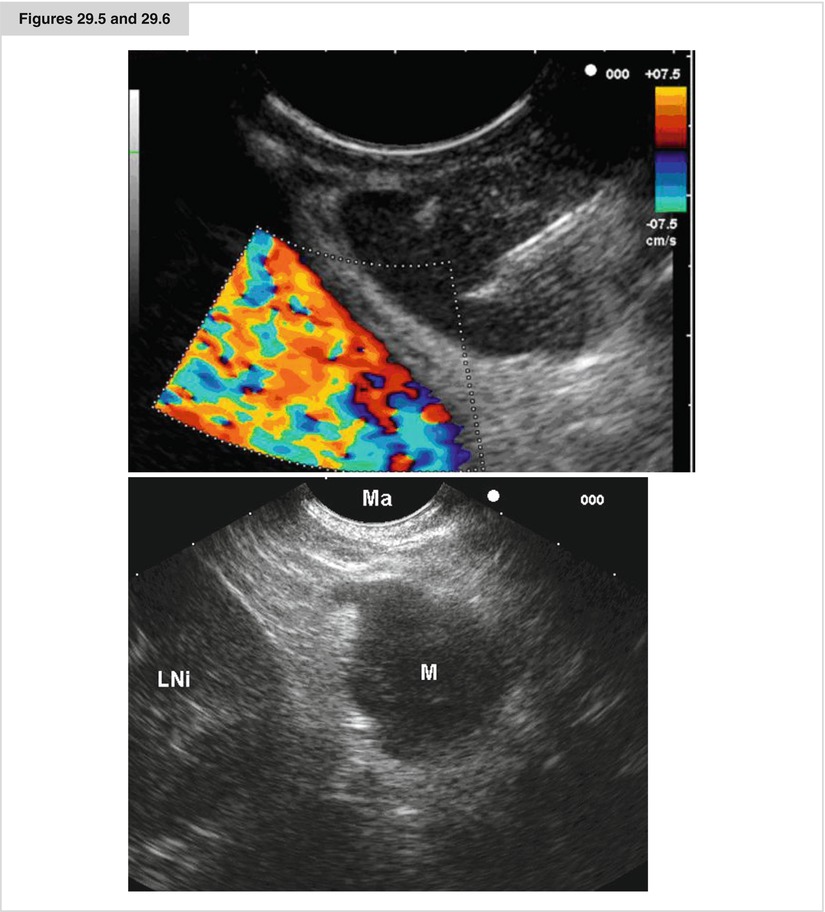
Figures 29.5 and 29.6
An EUS FNA procedure from a lymphnode in position 7, the descending aorta is shown by a doppler flow examination. THe needle reflex is seen in the node. An EUS image of an enlarged left adrenal (LNi left kidney, M adrenal, MA stomach)
Transbronchial needle aspiration. Merely a curiosity at its inception, flexible bronchoscopy has emerged as an essential diagnostic and therapeutic modality for a variety of lung diseases. The addition of transbronchial needle aspiration (TBNA) not only improved bronchoscopy’s diagnostic yield, it further extended the role of bronchoscopy in the evaluation of mediastinal pathology and in the diagnosis and staging of bronchogenic carcinoma. The first description of mediastinal lymph node sampling through the tracheal carina using a rigid bronchoscope was by Schieppati. In 1978, Wang et al. demonstrated that it was feasible to sample paratracheal nodes using TBNA. Subsequent publications highlighted the use of this technique in diagnosing endobronchial and peripheral lesions and the ability of TBNA to provide a diagnosis even in the absence of endobronchial disease. The diagnostic yield of TBNA in assessing hilar–mediastinal lymph node involvement in lung cancer varies greatly in the published literature, from 15 to 85 %. Recently, a meta-analysis of TBNA for the mediastinal staging of non–small cell lung cancer (NSCLC) demonstrated that TBNA is highly specific for identifying mediastinal metastases, whereas the sensitivity depends heavily on the study population under investigation. In studies including patients with a 34 % prevalence of mediastinal metastases, the sensitivity was only 39 %, whereas in a population with an 81 % prevalence, it was 78 %. Today, TBNA is increasingly combined with rapid onsite cytopathologic examination of the smears, as several papers demonstrated. Despite the impact of TBNA on patient management, surveys demonstrate that only 10–30 % of pulmonologists regularly use this technique. The main reasons for its limited use are a lack of needle monitoring, difficulties in performing the procedure, and a belief, despite the evidence in the literature, that TBNA is not useful (Wang et al. 1978).
Endobronchial ultrasound. The integration of ultrasound technology and flexible fiber bronchoscopy enables the imaging of lymph nodes, lesions, and vessels located beyond the tracheobronchial mucosa. Developed in 2002, the endobronchial ultrasound (EBUS) bronchoscope (Olympus BF-UC160F-OL8 or BF-UC260F-OL; Olympus America, Center Valley, PA) looks like a normal broncho-videoscope but is 6.9 mm wide and has a 2-mm instrument channel and a 30° side viewing optic. Furthermore, a curved linear array ultrasonic transducer sits on the distal end and may be used either with direct contact to the mucosal surface or via an inflatable balloon, which may be attached at the tip. This allows a conventional endoscopic picture side by side with the ultrasonic view. Ultrasonic scanning is performed at a frequency of 7.5–12 MHz with tissue penetration of 20–50 mm. An ultrasound processor processes the ultrasonic image. EBUS allows the bronchoscopist to visualize airway structures as well as surrounding processes. It is very valuable for staging advanced cancer with regard to intramural or nodal spread. EBUS can identify N1, N2, and N3 nodes without surgical intervention, reducing the need for expensive surgery (Herth et al. 2006a)
Endobronchial ultrasound (continued). The actual TBNA is performed by direct transducer contact with the wall of the trachea or bronchus. When a lesion is outlined, a 21-gauge needle (NA-201SX-4022; Olympus Corporation, Tokyo, Japan) may be advanced through the working channel and lymph nodes can be punctured under real-time ultrasound visualization. The needle has an internal sheath to prevent contamination during biopsy. At the same time, color Doppler may be used to identify surrounding vascular structures. Once the target lymph node or mass has been clearly identified with EBUS, the needle is inserted in the lesion under real-time ultrasound guidance. Suction is applied with a syringe, and the needle is moved back and forth inside the lesion. The stylet of the needle is left in place on the first puncture to minimize bronchial cell contamination; once the needle tip is inside the target tissue, the stylet is removed. We stab the target 10–15 times without suction and apply suction only for the last two or three stabbing motions. Prior to retracting the needle into the needle sheath, suction must be removed to minimize sample loss into the syringe. The specimen is then air-flushed on a slide, the needle is flushed with heparin–saline solution to avoid clotting, and the procedure is repeated three times at every lymph node station. Lymph node stations that can be reached via EBUS are the highest mediastinal (station 1), upper paratracheal (2L and 2R), lower paratracheal (4R and 4L), subcarinal (station 7), hilar (station 10), interlobar (station 11), and lobar nodes (station 12). The highest-staging node should be biopsied first; otherwise, the needle must be changed each time. Lymph nodes 5 mm and larger can be sampled successfully and, to date, have shown excellent diagnostic yield. The number of mediastinal lymph node stations to sample depends on the purpose of the examination. Clearly, NSCLC staging requires sampling of at least three stations (4R, 4L, and 7). Every attempt should be made to sample nodes at these sites, even if size and ultrasonographic features are normal. At our institution, we routinely sample mediastinal lymph nodes that are 5 mm or larger in short-axis diameter. The learning curve for EBUS-TBNA has been evaluated and recorded from 10 procedures to achieve excellent sensitivity and diagnostic accuracy. In a recently published meta-analysis, EBUS-TBNA was shown to have a high-pooled sensitivity of 93 % and specificity of 100 %. Multiple publications have shown that even in patients with lymph nodes under 1 cm (which had been termed N0 by CT criteria), the use of EBUS-TBNA could still show a large percentage of patients with N2/N3 disease (some despite also being negative on PET-CT). Complications such as bleeding or infection are very rare and have been reported only as case reports (Adams et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


