Figure 9.1
The modern rigid bronchoscope—essentially a straight, hollow stainless steel tube—has not changed much in the past 100 years. It is available in various lengths, and diameters range from 5 to 14 mm. The internal diameter is uniform throughout, and the distal ends of most bronchoscopes are beveled. Through slits in the distal wall, it is possible to ventilate the contralateral side during intubation of the main bronchus. The proximal end of the bronchoscope consists of a central opening and various side ports for connection of jet ventilation or conventional ventilation devices, as well as being a source of illumination. Rigid bronchoscopy usually is performed under general anesthesia with short-acting agents, such as propofol. Ventilation during the procedure may be achieved via conventional or jet ventilation. Jet ventilation may be applied to open airways and when connected to the rigid scope directly into the trachea. The advantage of jet ventilation is the free space left for procedures. If expiration is obstructed, jet ventilation carries an increased risk of barotrauma. After induction of general anesthesia, the patient’s neck is partially hyperextended and the bronchoscope is introduced in the midline with the bevel facing anteriorly. With the bronchoscopist’s thumb protecting the patient’s upper teeth, the bronchoscope is advanced under the epiglottis and rotated 90° to pass through the vocal cords. Once the trachea is entered, the bronchoscope is rotated back through 90° and advanced to the lower trachea. To access one of the main bronchi, the patient’s head must be rotated toward the contralateral shoulder. A charge-coupled device chip video camera is connected to the eyepiece for visualization on a monitor and to allow recording of the procedure
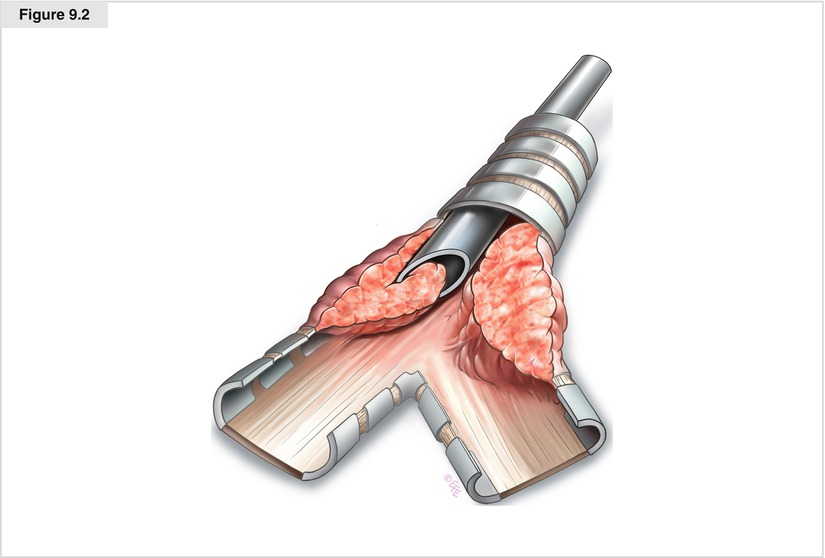
Figure 9.2
Various accessory instruments, including different types of forceps, suction catheters, and laser fibers, are used during bronchoscopy by advancing them via the proximal opening. The larger-bore rigid bronchoscopes allow optical systems and diagnostic and/or therapeutic tools to pass through the scope simultaneously. Rigid bronchoscopy often is preferred for removing foreign bodies or debulking endoluminal tumors because the airway is better protected and larger tools can be used for grasping. The distal ends of most bronchoscopes are beveled, allowing atraumatic passage through the vocal cords. Hence, they also may be used as a tool for managing endobronchial disease. The edge of the bronchoscope allows the shearing off of large sections of endobronchial tumor from the airway wall in a technique often referred to as apple coring. Although this procedure is technically difficult, apple coring combined with the use of larger biopsy forceps allows tumor to be resected quickly from the obstructed airway. Not only can the bevel be used to core out tumor, but corkscrewing through tight scar stenoses also is feasible. This procedure is very fast and helpful in life-threatening obstructive stenosis of the trachea
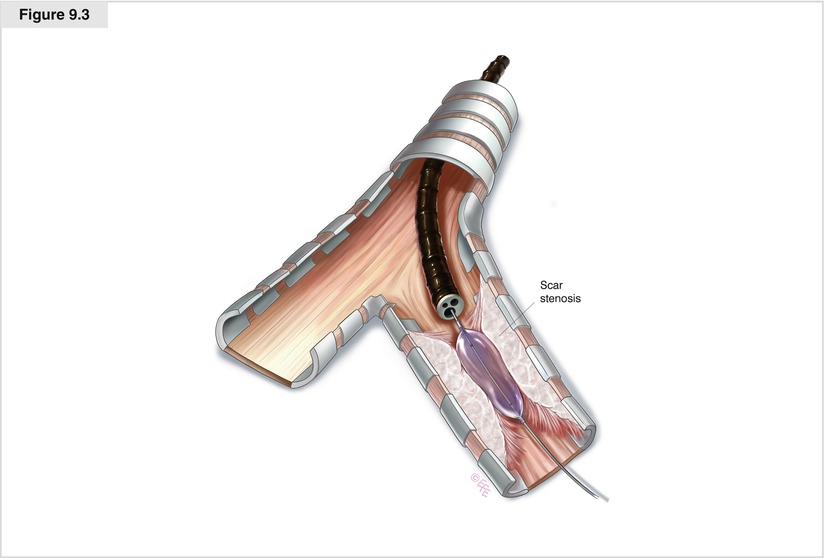
Figure 9.3
Dilatation of a scar stenosis can be achieved with controlled insertion of the barrel of the rigid bronchoscope, the sequential introduction of serially enlarging semi-rigid dilators, or balloon dilatation. Sequential balloon dilatation has the advantage of causing less mucosal trauma and subsequent granulation tissue than dilatation with the rigid bronchoscope. This technique has been used successfully in patients with airway stenosis following surgical resection of the airway and lung transplantation, in those with postintubation tracheal stenosis, and in those with malignant airway obstruction. The balloon is passed into the stenosis via either a rigid or flexible bronchoscope. The appropriate diameter and length of the balloon are chosen for the particular lesion. Ideally, 5–10 mm of balloon should extend beyond the stenosis both proximally and distally. To minimize the risk of tracheobronchial rupture, the treatment should be performed as a series of dilatations. Once the balloon is inflated to the prescribed pressure, this dilation pressure should be maintained for 1–2 min if the patient can tolerate this without discomfort or hypoxia. Today, three-stage balloons for a stepwise dilatation are available. Balloon dilatation is particularly effective in benign scar stenosis, preparing stenotic airways for stent placement, and expanding stents after insertion. Dilatation is immediately effective for intrinsic and extrinsic compression, but results typically are not sustained. In fact, mucosal trauma may lead to granulation and accelerate restenosis. The main risk of balloon bronchoplasty is airway rupture, rarely resulting in pneumothorax or pneumomediastinum, mediastinitis, and bleeding
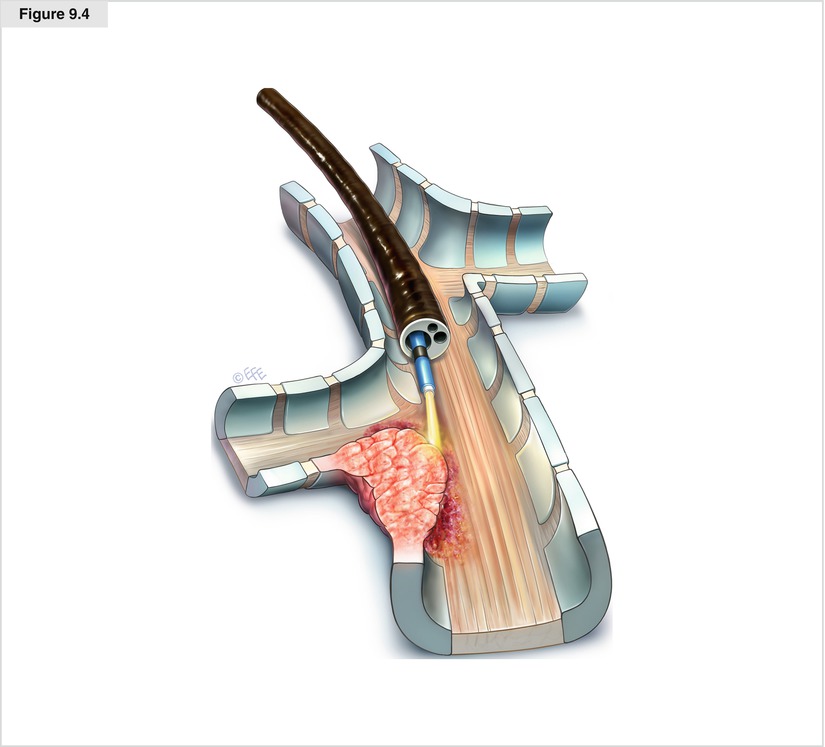
Figure 9.4




Endobronchial electrocautery and argon plasma coagulation (APC) are modes of thermal tissue destruction. Similar to laser tissue destruction, the effect of both electrocautery and APC is determined by heat and tissue interaction, and is fairly rapid. Application of high-frequency electric currents creates heat that coagulates or vaporizes tissue. Depending on the energy used, either coagulation or vaporization can be induced. During APC, a neutral plate must be attached to the patient to ground currents. Argon plasma is formed when a spark created at the tip of the probe ionizes argon gas. The plasma then finds the nearest grounded tissue and produces coagulative necrosis. APC causes a more homogeneous, but superficial, thermal effect because of its noncontacting nature and lower energy density. During endoscopic procedures, a coagulation depth of 2–3 mm may be achieved. The black ring of the probe should always be visible in the endoscopic view to avoid damage to the distal end of the bronchoscope. The energy setting is usually between 25 and 40 W. Energy settings can be changed depending on the visible tissue effect. During the procedure, it is important to apply continuous suction for the target area to remain free of blood and mucus as well as to evacuate smoke created during coagulation. In addition to the risks associated with rigid or flexible bronchoscopy, potential complications are similar to those of other thermal therapies and include airway fires, hemorrhage, airway perforation, and stenosis. To avoid airway fire, the inspired oxygen should be reduced as low as possible, preferably to a fraction of inspired oxygen (Fio 2) less than 40 %
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


