Achalasia subtype
Manometry criteria
Type I (classic)
Impaired EGJ relaxation (IRP >10 mmHg)
Absent peristalsis
No significant esophageal pressurization
Type II (with compression)
Impaired EGJ relaxation (IRP >15 mmHg)
Absent peristalsis
≥20 % swallows with panesophageal pressurization to >30 mmHg
Type III (spastic)
Impaired EGJ relaxation (IRP >17 mmHg)
Absent peristalsis
≥20 % swallows with premature contractions (distal latency <4.5 s)
EGJ outflow obstructiona
Impaired EGJ relaxation (IRP >15 mmHg)
Some preserved weak or normal peristalsis
The other absolute requirement to establish a diagnosis of achalasia is inclusion of an imaging study (usually endoscopy) to rule out pseudoachalasia. Upper endoscopy can help determine the degree of esophageal dilatation, whether or not there is significant esophageal retention of food and fluid, and evaluate for Candida esophagitis. A barium esophagram is also often done and may help in instances where there are equivocal manometric findings or when the manometry catheter cannot be passed into the stomach due to severe esophageal dilatation and angulation. The esophagram can also quantify the degree of esophageal emptying if done as a “timed barium esophagram” protocol (200 ml of barium with upright images at 1, 2, and 5 min). Endoscopic ultrasound and/or CT may be necessary when suspicion of pseudoachalasia is high.
Endoscopic Management
There are three endoscopic options for achalasia that merit discussion: botulinum toxin injection, pneumatic dilation, and per-oral endoscopic myotomy. In general, medical therapy with smooth muscle relaxants is ineffective and should be reserved for patients with substantial comorbidity making them poor risks for anesthesia and/or surgery. Patients who are judged fit for general anesthesia should be counseled to pursue a definitive treatment capable of alleviating EGJ outflow obstruction such as endoscopic pneumatic dilation, endoscopic surgical myotomy, or laparoscopic Heller myotomy. Surgical myotomy will be discussed in the subsequent chapter of this text.
Endoscopic Injection of Botulinum Toxin
The standard protocol for endoscopic botulinum toxin (Botox) injection into the LES is to inject 100 units with a sclerotherapy needle about 1 cm proximal to the squamocolumnar junction in four radially dispersed aliquots. Using this technique, Pasricha reported improved dysphagia in 66 % of achalasia patients for 6 months [14]. Botox prevents acetylcholine release at cholinergic synapses thereby negating the effect of these nerves on the sphincter. The physiologic effect is eventually reversed by axonal regeneration and most patients who derive benefit from the procedure relapse and require retreatment within 12 months. However, there have been reports that repeated treatments result in fibrosis of the sphincter making subsequent Heller myotomy more challenging [15–17]. Recognizing these limitations, Botox injection should not be utilized as a first-line therapy for achalasia for most patients. Rather, it should be reserved for poor surgical candidates and special circumstances.
Pneumatic Dilation
An achalasia dilator is a noncompliant, cylindrical balloon that is positioned across the LES and inflated with air using a handheld manometer. The only design currently available in the USA, the Rigiflex dilator, is positioned fluoroscopically over a guidewire and is available in 30, 35, and 40 mm diameters. Bougie and standard through-the-scope balloon dilators (maximal diameter of 20 mm) have no sustained efficacy in achalasia and should not be used. A cautious approach to dilation with the Rigiflex dilators is to initially use the 30 mm dilator and follow with a 35 mm dilator 2–4 weeks later if the initial dilation was insufficient. The reported efficacy of pneumatic dilation ranges from 32 to 98 % [18]. Patients with a poor result or rapid recurrence of dysphagia are unlikely to respond to additional dilations, but subsequent response to myotomy is not influenced. The major complication of pneumatic dilation is esophageal perforation. Although the reported incidence of perforation from pneumatic dilation ranges from 0 to 16 %, a recent systematic review on the topic concluded that using modern technique, the risk was less than 1 %, comparable to the risk of unrecognized perforation during Heller myotomy [19]. Furthermore, most perforations are clinically obvious and when surgically repaired within 6–8 h have outcomes comparable to patients undergoing elective Heller myotomy.
Although there is no standardized approach to the technique of pneumatic dilation, there are some basic principles that should be followed (Table 11.2). The patient should have appropriate dietary instructions before the procedure so that there is minimal residual food in the esophagus during the procedure. The balloon dilator is completely deflated prior to both passage and prior to withdrawal using a T-piece and large syringe to minimize trauma to the oropharynx. Pneumatic dilation requires concomitant endoscopy and fluoroscopy to place and visualize the guidewire and to verify appropriate balloon position. Our practice has been to use stiff spring-tipped Savary guidewires rather than the flimsy wires provided by the manufacturer. The balloon size is chosen using a graded approach, starting with a 30 mm balloon and increasing to the 35 mm size if patients do not respond. We do not recommend using the 40 mm balloon because of reports suggesting an unacceptable perforation rate. Accurate placement of the balloon is crucial to the effectiveness of the procedure, and this must be verified fluoroscopically during the initial stages of balloon inflation (Fig. 11.1). The inflation pressure of the balloon is not stipulated; full effacement of the sphincter on fluoroscopy is the endpoint of interest, which is usually associated with distention pressures of 8–15 psi. Patients should be observed in recovery for at least 2 h with careful assessment for post-procedure pain. A gastrografin/barium swallow study should be obtained if there is any worry of perforation. Patients should be explicitly advised to seek care emergently if they develop fever, shortness of breath, severe pain (especially if pleuritic), or subcutaneous emphysema.
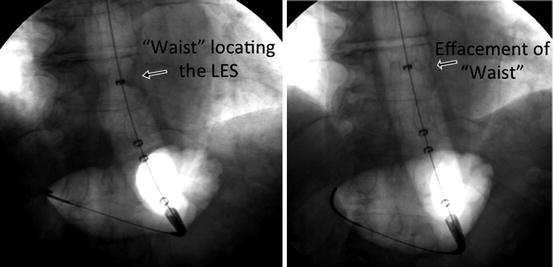
Table 11.2
Pneumatic dilation protocol. “Recommended” should be universally applied while there is no consensus among experts on “other suggestions”
Recommended | Other suggestions | |
|---|---|---|
Pre-dilation | N.P.O. ≥ 12 h | Clear liquids for 24–48 h |
Anesthesia | Same as for diagnostic EGD | MAC or general |
Dilator size selection | 30 mm unless previously unsuccessful, either within the past month or in prior treatment series | 35 mm balloon in young male patients |
Positioning | Localize the EGJ using fluoroscopy over a stiff guidewire | |
Balloon inflation | Slow inflation to capture the “waist” of the LES | Inflate balloon to at least 8 psi |
Deflate and reposition if the waist is not visible or is seen to migrate off the top of the balloon | ||
Maintain tension on the dilator during inflation to resist balloon getting “pulled” into the esophagus | ||
Time of inflation | One inflation, slowly increasing balloon pressure until the “waist” of the LES is seen to fully efface on fluoroscopy; then fully deflate, aspirate empty with a large syringe connected by a T-piece, position the patient on their side, and remove wire and dilator in unison | Inflate balloon for 15–60 s |
Repeat the dilation twice | ||
Post-procedure | Observe in recovery for at least 2 h | Routine contrast study |
Water-soluble contrast study prior to discharge if pain or other clinical parameters are concerning | ||
PRN pain medications | ||
2 weeks of PPI therapy | ||
Follow-up | Assess efficacy at 2–4 weeks, 6 months, and 12 months | Repeat dilation at shorter intervals (2–4 weeks) |
Repeat dilation with 35 mm dilator if treatment failure within 6 months |

Fig. 11.1
Fluoroscopic images taken during pneumatic dilation showing proper localization of the LES on the expanding balloon (left) and complete effacement of the sphincter (right)
Studies using pneumatic dilation as the initial treatment of achalasia have reported excellent long-term symptom control. However, a third of patients will relapse in 4–6 years and may require repeat dilation. Response to therapy may be related to preprocedural clinical parameters, such as age (favorable if age > 45), gender (female > male) [20], esophageal diameter (inversely related to response), and achalasia type (type II better than I and III) [5, 13]. Although surgical myotomy has a greater response rate than a single pneumatic dilation, it appears that a strategy utilizing a series of dilations with the potential for repeat is comparable to surgery and a reasonable alternative to surgery. A recent randomized controlled trial compared this type of graded strategy to surgical myotomy and found it to be non-inferior in efficacy [21].
Per-oral Endoscopic Myotomy (POEM)
Although laparoscopic Heller myotomy and pneumatic dilation are effective treatments for achalasia, some drawbacks exist with each. Consequently, there has been interest in developing a hybrid technique incorporating an endoscopic approach, but applying principles of a surgical myotomy. This technique termed per-oral endoscopic myotomy, or POEM, was initially described by Pashricha et al. [22] and subsequently developed by Inoue et al. in Japan (Fig. 11.2) [23].
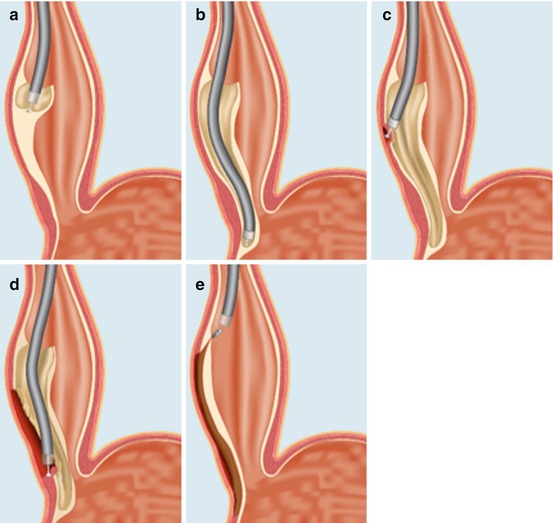

Fig. 11.2
Schematic of the POEM procedure (see text): (a) entry into the submucosal space, (b) submucosal tunnel to the gastric cardi, (c) beginning the myotomy, (d) completion of the myotomy, and (e) closing the mucosotomy with endoclips (Inoue et al. [23])
The procedure should be done in the operating room under general anesthesia (positive pressure ventilation) with CO2 endoscopic insufflation (Table 11.3). After preoperative intravenous antibiotics are given, diagnostic endoscopy should be done to rule out retained food or Candida esophagitis, as the presence of either should postpone the procedure. We also suggest tight blood pressure control (SBP ~ 100 mmHg) to help reduce submucosal bleeding. It is critical to turn off the air insufflation to avoid tension pneumomediastinum and subcutaneous emphysema.
Table 11.3
Per-oral endoscopic myotomy protocol. “Recommendations” should be universally applied
Recommendations | Other suggestions | |
|---|---|---|
Pre-procedure | N.P.O. ≥ 12 h | Nystatin S/S for 5 days |
Clear liquids for 48 h | ||
Intravenous antibiotics | ||
Anesthesia | General
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|