INDICATIONS/CONTRAINDICATIONS
The primary indication for surgical resection of esophageal cancer is for potential cure, which can be achieved in patients whose tumors are confined to the esophageal wall and when only limited local-regional disease is found. One should aim at maximizing the chance of an R0 resection (macroscopic and microscopic clearance of proximal, distal, and lateral margins), a parameter that has consistently been shown to be of major prognostic significance.
Increasingly neoadjuvant treatments including chemotherapy or chemoradiotherapy are used to treat esophageal cancer. Consistently, these strategies have been shown to result in a higher rate of R0 resection, and a pathologic complete response (in resected surgical specimen) can be achieved in approximately 10% for chemotherapy alone, and up to 30% for chemoradiotherapy. Although the benefits of neoadjuvant treatments over surgical resection alone are not proven in randomized controlled trials and remain controversial, these strategies are routinely used in many centers. In patients with advanced staged disease where the chance of an R0 resection is lower such as c-T3/T4 disease or those with multiple local-regional nodal spread, these therapies will result in significant downstaging in many patients, making a subsequent R0 resection possible. Surgery and especially en bloc esophagectomy after radiotherapy can be difficult; postirradiation fibrosis often obscures tissue planes. It is also uncertain if adherence to adjacent structures is related to residual tumor infiltration or merely desmoplastic reaction. This is especially important when minimally invasive surgical techniques are used. How surgical resection should be integrated into programs of multimodality treatment remains controversial and differs among institutions.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Accurate tumor staging should be performed to ensure maximal chance of R0 resection. This usually involves computed tomography (CT) scan, endoscopic ultrasound (EUS) ± fine-needle aspiration cytology of suspicious nodal metastases. Positron emission tomography (PET) scan is becoming the standard investigation at many institutions; it provides additional information on suspicious lesions on EUS and CT scans, especially of distant lesions. Postneoadjuvant staging is notoriously inaccurate. Tissue planes between the tumor and the adjacent structures are still best assessed by EUS, though accuracy decreases substantially after radiation.
Physiologic assessments are important to exclude patients for surgery, and optimization of cardiopulmonary function is of particular importance. Factors often cited as being predictive of morbidity and mortality after esophagectomy include advanced age, poor performance status, nutritional depletion and weight loss, more proximally located tumor, poor pulmonary function, cirrhosis, and abnormal cardiac evaluation. Patients with squamous cell cancers are more likely to be malnourished, have high alcohol intake, be smokers, and have more impairment of pulmonary and hepatic functions. Patients with adenocarcinomas on the other hand are more likely to be overweight and are more at risk from cardiovascular diseases.
Preoperative assessments include a detailed history and clinical physical examination, simple blood profiles, chest radiograph, electrocardiogram, and pulmonary spirometry. More detailed cardiac workup, including echocardiography, myocardial perfusion scans, or angiograms, are selectively applied when specific indications exist. Cirrhosis is not an absolute contraindication to esophagectomy, although the presence of esophageal varices usually contraindicates surgery.
In general, limited improvement can be made to a patient’s physiologic status. However the following measures should be instituted.
 Cessation of smoking and alcohol intake
Cessation of smoking and alcohol intake
 Incentive spirometry and chest physiotherapy
Incentive spirometry and chest physiotherapy
 Optimization of bronchodilator therapy in patients with asthma or significant chronic obstructive airway disease
Optimization of bronchodilator therapy in patients with asthma or significant chronic obstructive airway disease
 Consideration of coronary revascularization with angioplasty and coronary stenting in the presence of significant coronary ischemia. Antithrombotic medications, such as aspirin and clopidogrel, are often indicated after coronary intervention for a period of time. In such patients, it may be prudent to treat them with neoadjuvant therapy, so that time is not lost in waiting for an optimal time for surgery after coronary stenting
Consideration of coronary revascularization with angioplasty and coronary stenting in the presence of significant coronary ischemia. Antithrombotic medications, such as aspirin and clopidogrel, are often indicated after coronary intervention for a period of time. In such patients, it may be prudent to treat them with neoadjuvant therapy, so that time is not lost in waiting for an optimal time for surgery after coronary stenting
 In patients with high-grade esophageal tumor stenosis, a fine-bore nasogastric tube can be placed for nutritional support while workup is performed, and is preferable over parenteral nutrition, gastrostomy, or jejunostomy feeding
In patients with high-grade esophageal tumor stenosis, a fine-bore nasogastric tube can be placed for nutritional support while workup is performed, and is preferable over parenteral nutrition, gastrostomy, or jejunostomy feeding
 Diabetic control should be optimized
Diabetic control should be optimized
 Immediate preoperative preparations include prophylactic antibiotics to be given at anesthesia induction and deep vein thrombosis prophylaxis. Bowel preparation is not necessary, unless a colonic interposition is intended
Immediate preoperative preparations include prophylactic antibiotics to be given at anesthesia induction and deep vein thrombosis prophylaxis. Bowel preparation is not necessary, unless a colonic interposition is intended
 SURGERY
SURGERY
Many factors need consideration in choosing the approach to surgical resection. These include the following.
 The location of the intended anastomosis, whether it should be in the thorax or the neck
The location of the intended anastomosis, whether it should be in the thorax or the neck
 The route of reconstruction: Conduit placed in the thoracic cavity or to the neck via the posterior mediastinum, retrosternal, or subcutaneous route
The route of reconstruction: Conduit placed in the thoracic cavity or to the neck via the posterior mediastinum, retrosternal, or subcutaneous route
 The conduit used: Stomach, jejunum, or colon
The conduit used: Stomach, jejunum, or colon
The following description refers to the standard combined abdominal right thoracic approach for “en bloc” resection with lower mediastinal and upper abdominal lymphadenectomy using the stomach as the conduit with an intrathoracic esophagogastrostomy. Variations in the technique are briefly illustrated.
Positioning and Anesthetic Technique
The patient is first placed in the supine position for the abdominal part of the surgery. To prepare for the subsequent right thoracotomy, a double-lumen endotracheal tube is usually placed. This, however, can be placed after the abdominal phase of the operation to lessen the duration of airway trauma in using this relatively large endotracheal tube. At the author’s institution, it is routine practice to place a single-lumen endotracheal tube, and during the thoracotomy phase, a right bronchial blocker is placed. This results in less airway trauma and the more flexible tube allows better exposure of areas behind the trachea and also around the left main bronchus. In the thoracic phase of surgery, the patient is placed in a full lateral decubitus position with the right arm approximately at a right angle at the shoulder.
Operating Technique: Abdominal Phase
A midline or a bilateral subcostal incision is made. The author prefers the latter because it gives improved exposure to the upper abdomen especially in obese patients. The stomach is mobilized together with celiac trifurcation lymphadenectomy via the following steps.
 The gastrocolic omentum is taken off the greater curvature of the stomach, preserving the right gastroepiploic vessels and arcades. Complete omentum resection is not necessary. Division of the gastrocolic omentum can be carried out just outside the right gastroepiploic vessels. The left crus is exposed when the short gastric vessels are divided and the fundus mobilized medially (Fig. 22.1). The phrenoesophageal membrane is detached, and the abdominal esophagus and cardia can be freed on the left side.
The gastrocolic omentum is taken off the greater curvature of the stomach, preserving the right gastroepiploic vessels and arcades. Complete omentum resection is not necessary. Division of the gastrocolic omentum can be carried out just outside the right gastroepiploic vessels. The left crus is exposed when the short gastric vessels are divided and the fundus mobilized medially (Fig. 22.1). The phrenoesophageal membrane is detached, and the abdominal esophagus and cardia can be freed on the left side.
 The gastrohepatic ligament is then detached from the liver and from the portal structures. Dissection from the right side toward the esophageal hiatus frees the right crus, and dissection anterior to the esophagus will meet the already dissected plane from the left. The anterior vagus nerve can be divided at this point. The esophagus is thus freed on both sides as well as anteriorly. A sling placed around the lower esophagus, such as a Penrose or latex drain, may help later dissection by providing retraction.
The gastrohepatic ligament is then detached from the liver and from the portal structures. Dissection from the right side toward the esophageal hiatus frees the right crus, and dissection anterior to the esophagus will meet the already dissected plane from the left. The anterior vagus nerve can be divided at this point. The esophagus is thus freed on both sides as well as anteriorly. A sling placed around the lower esophagus, such as a Penrose or latex drain, may help later dissection by providing retraction.
 For a tumor of the distal esophagus located at the hiatal opening, especially for a transmural T3/T4 tumor, a cuff of diaphragmatic crura can be removed together with the tumor. When a cuff of muscle from the esophageal hiatus is removed, both pleural cavities are likely to be entered, but this is of no serious consequence.
For a tumor of the distal esophagus located at the hiatal opening, especially for a transmural T3/T4 tumor, a cuff of diaphragmatic crura can be removed together with the tumor. When a cuff of muscle from the esophageal hiatus is removed, both pleural cavities are likely to be entered, but this is of no serious consequence.
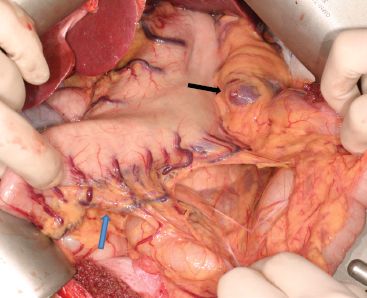
Figure 22.1 Figure showing detachment of the gastrocolic omentum off the stomach. It is not necessary to perform a total omentectomy. Division of the gastrocolic ligament a short distance from the right gastroepiploic arcade is sufficient. This is the most important blood supply to the gastric conduit. The left gastroepiploic artery is ligated near the inferior pole of the spleen. Blue arrow, right gastroepiploic arcade; Black arrow, inferior pole of spleen where the left gastroepiploic artery will be found.
 The stomach is then reflected upward and dissection is begun at the celiac trifurcation (Fig. 22.2). Using fine electrocautery, dissection is performed along the anterior aspect of the common hepatic artery. Lymphadenectomy can then proceed laterally toward the hepatoduodenal ligament. It is sufficient to remove nodes along the anterior surface of the common hepatic artery. Medially toward the origin of the left gastric artery at the celiac axis, the left gastric artery and coronary vein require ligation (Fig. 22.3). The origin of the left gastric artery is identified separately as it comes off the celiac axis and it is doubly ligated and cut between ligatures (Fig. 22.4). Further dissection toward the left will clear the lymphatic tissues on the splenic artery. The areolar tissue superior to the common hepatic artery and splenic artery is therefore cleared en bloc with the abdominal esophagus toward the hiatus.
The stomach is then reflected upward and dissection is begun at the celiac trifurcation (Fig. 22.2). Using fine electrocautery, dissection is performed along the anterior aspect of the common hepatic artery. Lymphadenectomy can then proceed laterally toward the hepatoduodenal ligament. It is sufficient to remove nodes along the anterior surface of the common hepatic artery. Medially toward the origin of the left gastric artery at the celiac axis, the left gastric artery and coronary vein require ligation (Fig. 22.3). The origin of the left gastric artery is identified separately as it comes off the celiac axis and it is doubly ligated and cut between ligatures (Fig. 22.4). Further dissection toward the left will clear the lymphatic tissues on the splenic artery. The areolar tissue superior to the common hepatic artery and splenic artery is therefore cleared en bloc with the abdominal esophagus toward the hiatus.
 Continued dissection upward can be carried out through the esophageal hiatus along the front of the aorta. Areolar tissues are freed from the aorta and remain attached to the resected specimen. The cardia and the abdominal esophagus are thus freed totally.
Continued dissection upward can be carried out through the esophageal hiatus along the front of the aorta. Areolar tissues are freed from the aorta and remain attached to the resected specimen. The cardia and the abdominal esophagus are thus freed totally.
 On the lesser curvature the right gastric vessels are divided at the angular incisura. One linear stapler is used to transect the stomach from this point upward toward the fundus. This is the first of a series of staplers used to transect the stomach to make a narrow gastric conduit. The application of one stapler at this stage of the operation will make the subsequent use of further staplers during the thoracic phase easier (Fig. 22.5).
On the lesser curvature the right gastric vessels are divided at the angular incisura. One linear stapler is used to transect the stomach from this point upward toward the fundus. This is the first of a series of staplers used to transect the stomach to make a narrow gastric conduit. The application of one stapler at this stage of the operation will make the subsequent use of further staplers during the thoracic phase easier (Fig. 22.5).
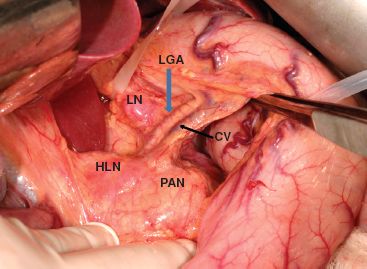
Figure 22.2 Figure showing the celiac axis to be dissected. CV, coronary vein; LGA, left gastric artery; LN, enlarged lymph node next to left gastric artery; HLN, enlarged hepatic artery lymph node; PAN, pancreas.



