Fig. 2.1
Cardiac sympathetic control
2.2.6 Effect of Sympathetic Stimulation on Action Potential Duration Restitution
Substantial evidence links enhanced sympathetic activation with ventricular arrhythmias and sudden cardiac death. Destabilisation of ventricular wave fronts leading to degeneration ventricular tachycardia into ventricular fibrillation appears to be related to the restitution properties of action potential duration. Restitution is described as the change in APD in response to the preceding diastolic interval, and steeply sloped restitution curves with large changes in APD for relatively small changes in diastolic interval over a wide range of diastolic intervals have been associated with complex unstable dynamic rhythms. Sympathetic stimulation with epinephrine in porcine models increases the slope of ventricular APD restitution curves. This was confirmed in humans in whom stimulation with both adrenalin and isoproterenol increased the steepness of the slope of APD restitution curves, further demonstrating the known effects of adrenergic stimulation in facilitating ventricular fibrillation (Taggart et al. 1990, 2003; Schwartz et al. 1988a, b; Hohnloser et al. 1994; Rosenshtraukh et al. 1994).
2.3 Cardiac Parasympathetic Nervous System Dysfunction as Manifested by Baroreflex Sensitivity and Heart Rate Variability
As mentioned earlier, the loss of protective vagal reflexes is associated with ventricular arrhythmias in heart failure and MI. Depressed baroreflex sensitivity (BRS) and heart rate variability (HRV), reflections of parasympathetic innervations, have been associated in humans and animal models of MI with a greater susceptibility to ventricular fibrillation during and after ischaemic episodes. Heart rate variability primarily reflects tonic vagal activity, whereas BRS measures predominantly reflex vagal activity in response to stressors. Middle-aged healthy men with high resting heart rates (>75 beats per minute) had a 3.8-fold increase in the risk of SCD compared with those with low basal heart rates (<60 beats per minute), with the risk of SCD increasing linearly with increasing resting heart rates over 23 years of follow-up, suggesting that high parasympathetic tone is protective against SCD (Ferrara et al. 1987; Schwartz et al. 1984; Issa et al. 2005b; Mannheimer et al. 1993).
2.3.1 Heart Rate Variability
Beat to beat, heart rate is not completely regular and is based in part on the autonomic innervation of the sinus node. This can serve as non-invasive marker of autonomic input to the heart, and the analysis can be accomplished in time or frequency domains. High frequencies are thought to represent the parasympathetic component of the autonomic nervous system, whereas low frequencies are mediated by both the sympathetic and parasympathetic nervous system and are affected by BRS. Very-low frequencies are influenced by many factors including the renin–angiotensin system and thermoregulation. This measurement is limited by its inherent use of sinus node innervation as a surrogate for ventricular parasympathetic innervation. In dog models of MI, Hull et al. (1990) showed that dogs who developed ventricular fibrillation had a significant decrease in all measures of HRV, demonstrating a high sensitivity and specificity of HRV in predicting susceptibility to ventricular arrhythmias. These studies were further confirmed by Adamson et al. who also showed that low-risk dogs recovered HRV after MI, whereas high-risk dogs continued to have depressed HRV parameters. Similar results were obtained in humans. Twenty-four-hour Holter recordings in post-MI patients showed that depressed HRV was a significant predictor of mortality after adjusting for clinical and demographic features, including ejection fraction (EF) (Farrell et al. 1991). These studies were further confirmed by other in post-MI patients, showing that impaired HRV was an independent predictor of cardiac mortality only within 6 months of MI and seemed to improve over time. That HRV improves over time is consistent with the decreasing risk of SCD after MI over a similar period. One of the largest of these trials involved 808 patients who underwent HRV analysis using 24-h Holter monitors 11 ± 3 days post-acute MI. In univariate analysis, HRV below 50 ms imposed a hazard relative risk of 5.3, compared with patients with HRV above 100 ms, and remained a significant predictor of mortality after adjusting for clinical and demographic characteristics, other Holter features, and ejection fraction during a mean follow-up of 31 months. Of note, decreased HRV parameters have also been reported in patients with idiopathic dilated cardiomyopathy with history of sudden cardiac death compared with those without a history of ventricular tachyarrhythmias (Odemuyiwa et al. 1994; Kleiger et al. 1987; Schwartz et al. 1988b; La Rovere et al. 1998).
2.3.2 Baroreflex Sensitivity
The arterial baroreceptor control of the heart is generally studied using three techniques: (1) increasing blood pressure with vasoconstrictors such as phenylephrine and analysing heart rate response, this method is used most commonly; (2) lowering blood pressure with vasodilators such as nitroprusside to test reflex sympathetic tone; and (3) direct stimulation of carotid baroreceptors with neck suction. Just as in HRV, BRS was shown to be reduced after MI and to predispose to ventricular fibrillation first in dog MI models. These studies were carried forward to humans, where BRS was found to be lower in patients after MI than in control subjects, but the reduction was transient and appeared to return to baseline levels within 3 months, similar to the improvement seen in HRV and decreasing risk of SCD. The potential prognostic value of BRS was established in several human studies showing that a severely depressed BRS (<3 ms/mmHg) was associated with high mortality due to a high risk of arrhythmic events. The largest of these was the Autonomic Tone and Reflexes After Myocardial Infarction (ATRMI) study, a multicentre prospective trial of 1,028 patients who underwent HRV and BRS analysis within 1 month after MI. During 21 months of follow-up, low values of either heart rate variability (SDNN <70 ms) or BRS (<3.0 ms/mmHg) carried a significant multivariate risk of cardiac mortality (3.2 [95 % CI, 1.42–7.36] and 2.8 [95 % CI, 1.24–6.16], respectively). The association of low standard deviation of normal RR intervals (SDNN) and BRS further increased risk with the 2-year mortality being 17 % when both were low and 2 % when both were well preserved (SDNN >105 ms, BRS >6.1 ms/mmHg). In patients with EF above 35 % after MI, depressed BRS (<3.0 ms/mmHg) has identified, independently of age and EF, a subgroup of patients at long-term high risk of cardiovascular mortality (HR, 11.4 [95 % CI, 3.3–39]) who may benefit from more aggressive preventive strategies. Of note, BRS is improved in patients with MI who receive thrombolytic therapy or revascularisation compared with those treated conservatively (Taggart et al. 2003; Schwartz et al. 1988a).
2.4 Parasympathetic Modulation of Sudden Death: BRS Versus HRV
Although both HRV and BRS have been shown to be abnormal in heart failure and in post-myocardial infarction patients, the correlation between the two is only moderate (R = 0.63). This is consistent with the fact that HRV and BRS are different measures of parasympathetic activity, with HRV measuring tonic vagal activity over a 24-h period, whereas BRS is equivalent to a vagal response or variability stress test. Furthermore, BRS in some studies has been a stronger predictor of ventricular tachyarrhythmias than HRV, suggesting that measurements of the dynamic nature of the parasympathetic system may provide superior prognostic information. The underlying mechanisms of the protective effects of the parasympathetic nervous system are not well understood. Loss of vagal innervation, similar to sympathetic innervation, occurs as early as 5–20 min after coronary occlusion. Vigorous vagal activation during acute myocardial ischaemia has been shown to be protective against ventricular fibrillation in anaesthetised cats. Vagal stimulation in these animals after coronary artery ligation increases ventricular repolarisation by increasing levels of pertussis toxin – sensitive G protein – and reduces the risk of ventricular fibrillation. This reduction in risk is no longer observed if vagal stimulation is blocked by atropine or pertussis toxin. The antifibrillatory effects of vagal activation is confirmed by the prevention of ventricular fibrillation during acute ischaemia in dogs susceptible to sudden cardiac death by direct stimulation of the right vagus. In animal studies, direct muscarinic and vagal nerve stimulation with carbacholine, cyclic guanine monophosphate (cGMP), neostigmine, or oxotremorine or even indirect increase with exercise have been shown to reduce the incidence of ventricular tachyarrhythmias in dog infarct models of sudden cardiac death. Based on these studies, low-dose scopolamine was used in humans and was shown to increase HRV and BRS in healthy and in post-MI patients. Endurance exercise training in healthy human subjects also leads to an increase HRV in healthy subjects, suggesting increases in vagal tone. Whether these changes translate into improved mortality and decreased risk of ventricular arrhythmias remains unclear. Post-myocardial infarction dogs treated with low-dose scopolamine compared with controls continued to have a high risk of sudden cardiac death and recurrent ventricular fibrillation despite improvement in HRV parameters. Thus, interventions that improve vagal tone may not provide antifibrillatory effects and those that improve reflex tone may prove to be better targets for reducing the risk of ventricular arrhythmias. Until the cellular mechanisms of the protective effects of vagal innervation are understood, targeting the parasympathetic nervous system in ischaemic cardiomyopathy and prevention of sudden cardiac death will prove difficult (Farrell et al. 1991; Odemuyiwa et al. 1994; Kleiger et al. 1987; Schwartz et al. 1988b).
2.5 Clinical Methods of Assessing Autonomic Innervation in the Heart
One of the limitations in studies of the ANS and its relationship to cardiac electrophysiology has been the limited options available to study autonomic innervation of the heart. Specifically, determining where ganglia are located via an interventional approach or calculating nerve density at the neural-myocardial interface depends on surrogate markers that may not always be reproducible or offer the degree of fine-tuned data needed during ablation procedures. The most commonly used methods include nuclear imaging of sympathetic innervation, high-frequency stimulation, and complex fractionated electrograms (Taggart et al. 2003; Schwartz et al. 1988a). However, each has its limitations and has not been rigorously correlated with anatomic–pathologic studies which would be the gold standard for determining where ganglia are located and the density of sympathetic innervation. In turn, the ex vivo and open heart animal studies that have been done to date need to be translated into clinically useful electrophysiologic mapping procedures. To achieve this, either advances in available catheters or mapping systems or the development of novel agents that can highlight locations of ganglionated plexuses may be needed. Both the sympathetic and parasympathetic nervous systems are intricately involved in the modulation of cardiac excitability and arrhythmias. Neural remodeling with decrease in parasympathetic input, along with heterogeneous sympathetic denervation followed by hyperinnervation in addition to the observed structural remodeling of the diseased heart, creates the electrophysiologic substrate necessary to initiate and maintain arrhythmias. Only by a better understanding of the cellular and electrophysiologic mechanisms underlying normal innervation and neural remodeling will the prevention of sudden cardiac death become feasible (Sanderson et al. 1994; Eliasson et al. 1996; Issa et al. 2005a; Nademanee et al. 2000).
2.6 The Future of Therapeutic Approaches in Neurocardiology
The future of therapeutic approaches in neurocardiology lies both in novel treatment as in applying scientific integrative medical ideas that takes into account concurrent chronic degenerative and vascular disorders and interactions of multiple drug and nondrug treatments. In this respect, vagal stimulation, exercise training, electrical neurostimulation, music therapy, and renal denervation have become interesting options in the treatment of angina pectoris, heart failure, hypertension, and arrhythmias. As sympathetic tone is known to be increased and parasympathetic innervation decreased in cardiomyopathy patients, interventions that aim to reduce sympathetic tone and, therefore, increase parasympathetic tone should reduce the risk of sudden cardiac death and ventricular tachyarrhythmias (Fig. 2.2). This has, in fact, been shown to be true.
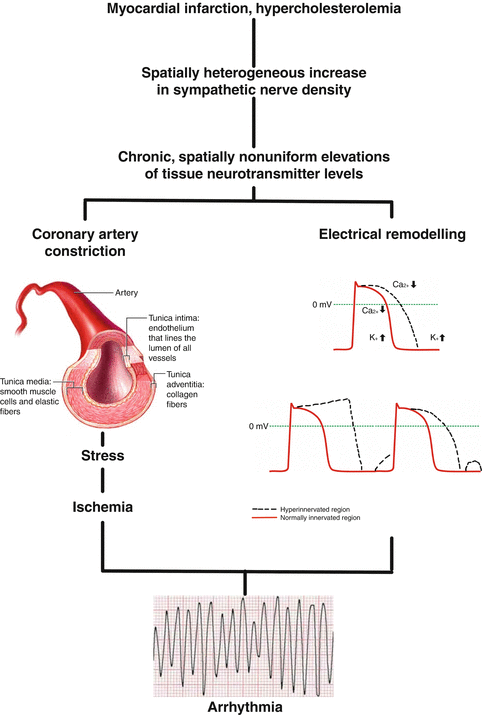

Fig. 2.2
Factors contributing to arrhythmogenesis in hearts with heterogeneous sympathetic innervation
2.6.1 Selective Sympathetic Blockade
In 1983, Schwartz et al. showed that the incidence of ventricular fibrillation was decreased from 66 % to zero by performing left stellectomy in post-MI dogs. Issa et al. demonstrated that thoracic spinal cord stimulation at T1–T2 segments reduced the incidence of ventricular tachyarrhythmias in a canine model of ischaemic cardiomyopathy from 59 to 23 % when applied during myocardial ischaemia. Furthermore, they observed a simultaneous decrease in heart rate and reduced systolic blood pressure, consistent with the antisympathetic effects of spinal cord stimulation. In a similar model, intrathecal clonidine, which is known to cause centrally mediated bradycardia and hypotension because of its sympatholytic effects when delivered via a catheter at T2–T4 spinal segments, also significantly reduced the occurrence of ventricular tachycardia and fibrillation during transient myocardial ischaemia. The report of sympathetic blockade in humans compared survival in a group of 49 patients with recurrent ventricular fibrillation (electrical storm) early after MI treated with standard advanced cardiac life support (ACLS) protocol vs. sympathetic blockade. Sympathetic blockade was established using left stellate ganglionic blockade in six patients and infusions of either propranolol or esmolol in 21 patients without antiarrhythmic therapy as recommended by ACLS. The 1-week and 1-year mortality were significantly higher in the group undergoing standard ACLS protocol, compared with the sympathetic blockade group (82 % vs. 22 % at 1 week, 95 % vs. 33 % at 1 year, respectively). Successful treatment of recurrent ventricular tachycardia, refractory to antiarrhythmic therapy, can be achieved by neuraxial modulation at the level of the spinal cord. The benefit of thoracic epicardial anaesthesia was reported in a patient with ischaemic cardiomyopathy and recurrent ventricular arrhythmia refractory to intubation and sedation, with the use of 0.25 % bupivacaine at T1–T2 interspace, reducing the number of ICD shocks from 86 in 48 h to zero (Sanderson et al. 1994).
Inhibiting sympathetic activity pharmacologically reduces the incidence of sudden cardiac death in patients with heart failure. In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), the aldosterone inhibitor eplerenone was associated with a clear reduction in sudden cardiac arrest in patients with acute MI complicated by left ventricular dysfunction. Beta-blockers and angiotensin-converting enzyme inhibitors have had the same effect. These findings indicate that adverse electrophysiologic consequences from sympathetic stimulation may contribute to the development of a proarrhythmic substrate and that antagonizing sympathetic activation can reduce the extent of adverse electrical remodeling to reduce the risk of sudden cardiac death (Nademanee et al. 2000).
2.6.2 Medical Therapies Modulating Cardiac Autonomics
As mentioned above, β-blockers, but also angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers, aldosterone antagonists, statins, and fish oil have been shown to decrease risk of SCD in ischaemic cardiomyopathy and significantly improve mortality. These classes of drugs have also been shown to modulate the autonomic nervous system to decrease sympathetic tone and/or increase parasympathetic tone.
Angiotensin II in the nucleus solitaire decreases baroreceptor reflex-evoked vagal bradycardia. Microinjection of angiotensin II into the nucleus of the solitary tract in rats significantly attenuates vagal output to the heart. This can be reversed with losartan, suggesting that ACEI and angiotensin receptor blockers may increase parasympathetic output to the heart, decreasing the risk of ventricular tachyarrhythmias. In humans, parasympathetic dysfunction, as measured by abnormal response to Valsalva manoeuvre and respiratory sinus arrhythmia, correlates with severity of heart failure. Treatment of non-ischaemic cardiomyopathy patients with enalapril for 4 weeks reverses these autonomic abnormalities. In experimental rat models of ischaemic cardiomyopathy, rats treated with the spironolactone derivative, canrenone, had decreased myocardial norepinephrine content (suggesting decreased hyperinnervation) and increased VF threshold. These antisympathetic effects were augmented if the rats also received ramipril concomitantly. As with ACEIs and aldosterone antagonists, statins also improve mortality in cardiomyopathy patients. Pliquette et al. showed that in rabbits with pacing-induced heart failure, statin therapy with simvastatin normalises sympathetic outflow and cardiovascular reflex regulation and showed a beneficial dose-dependent effect on baroreceptor sensitivity. The underlying mechanism for the beneficial effects of statin therapy in modulating the autonomic nervous system was further elucidated by recording renal sympathetic nerve activity and studying the effect of statins on angiotensin II type I gene expression and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity (a downstream protein activity by angiotensin II receptor activation) in the rostral ventrolateral medulla of rats. Simvastatin therapy significantly reduced angiotensin II-induced pressor and sympathoexcitatory responses, decreased baseline renal sympathetic nerve activity, and increased baroreceptor control of heart rate. Furthermore, simvastatin downregulated mRNA and protein expression of angiotensin II type I receptor and NADPH oxidase subunits in the medulla of heart failure rabbits. Lee et al. once more demonstrated hyperinnervation in rats with MI as shown by an increase in tyrosine hydroxylase and myocardial norepinephrine levels. But they subsequently went on to show that rats treated with pravastatin had lower arrhythmic scores in programmed electrical stimulation studies than controls not treated with a statin or treated with a K-channel blocker. Pravastatin seemed to mediate its antiarrhythmic effects by increasing KATP activity, as blocking of these potassium channels with the K-channel blocker glibenclamide reversed the beneficial effects of pravastatin. Fish oil has been shown to specifically decrease risk of sudden cardiac death in cardiomyopathy and post-MI patients. In elderly nursing home residents, supplementation with 2 g of fish oil significantly improved both high- and low-frequency components of HRV and SDNN, suggesting that fish oil can decrease sympathetic tone and increase parasympathetic response (Mannheimer et al. 1993; Sanderson et al. 1994; Eliasson et al. 1996; Issa et al. 2005a; Nademanee et al. 2000; Hjalmarson 1997).
2.6.3 Effect of Resynchronisation Therapy on Sympathetic Activity
Biventricular pacing has been shown to result in hemodynamic improvement in patients with depressed ejection fraction and intraventricular conduction delay. In patients with cardiomyopathy, biventricular pacing resulted in decreased sympathetic nerve activity along with improvement in blood pressure compared with intrinsic conduction in patients with left ventricular dysfunction and intraventricular conduction delay. Furthermore, in 50 patients implanted with biventricular pacemakers and randomised to therapy-on (n = 25) vs. therapy-off (n = 25), HRV was significantly improved in patients receiving resynchronisation therapy despite a lack of difference between mean atrial cycle length. Therefore, improvement in ventricular performance via resynchronisation therapy shifts the cardiac autonomic balance towards a more favourable profile of less sympathetic and more parasympathetic activation (Esler et al. 2010).
2.6.4 Vagal Function Mortality and Cardiovascular Risk
There are multiple measures that can be used to index activity of the vagus nerve. Resting HR, by virtue of being under tonic inhibitory control via the vagus, is a simple, inexpensive, and non-invasive measure of vagal function. The HR change following cessation of exercise is another measure that has been used to characterise vagal function. The decrease in HR after termination of exercise has been termed HR recovery and standardised methods have been developed for its assessment. Measures of heart rate variability (HRV) in both the time and frequency domains have also been used successfully to index vagal activity. In the time domain, the standard deviation of the inter-beat intervals (IBI), the percentage of IBI differences greater than 50 ms, and the mean square of the successive differences in IBIs (MSD) have been shown to be useful indices of vagal activity. In the frequency domain, both low-frequency (LF: 0.04–0.15 Hz) and high-frequency (HF: 0.15–0.40 Hz) spectral power have been used as indices of vagal activity. Whereas there is little contention concerning HF power reflecting primarily parasympathetic influences, LF power has been shown to reflect both sympathetic and parasympathetic influences. However, it is commonly reported that LF and HF are highly and significantly correlated. For example, we have recently reported that LF and HF power were positively correlated in both European American (r = 0.61) and African-American (r = 0.69) youths. Even larger correlations have been found between LF and HF in some large epidemiological studies (n = 11,654; r = 0.76). Thus, LF power often reflects substantial parasympathetic influence. This is not surprising given that parasympathetic influences are present over the whole range of the HRV spectrum, whereas the sympathetic influences roll off at about 0.15 Hz. In addition, measures of baroreflex sensitivity (an index of the responsiveness of the cardiovascular system to changes in blood pressure) have also been shown to be useful indicators of vagal function. The literature linking these different indices to morbidity and mortality is extensive. Importantly, whereas there are some differences among studies, the consensus is that lower values of these indices of vagal function are associated prospectively with death and disability. We will review some of these studies here to illustrate the range and power of the association between vagal function and cardiovascular disease and mortality. The studies related to mortality are listed in Table 2.1 and the studies related to risk factors are listed in Table 2.2. However, we should be clear that this is illustrative and not exhaustive.
Table 2.1
Studies of vagal function and mortality
Studies (1st AU) | Subject and sample size | Measures employed | Controlled variables | Relative risk |
|---|---|---|---|---|
Habib et al. (1999) | Over 30,000 men and women | HR | Gender and ethnicity | Threefold greater risk if HR > 90 bpm |
Relative those with HR < 60 bpm | ||||
Cole et al. (1999) | N = 2,428; 63 % men | HR recovery | Age, sex, the use or nonuse of medications, the presence or absence of myocardial perfusion defects on thallium scintigraphy, standard cardiac risk factors, resting heart rate, the change in heart rate during exercise, workload achieved | Unadjusted: not stated |
Adjusted: 4.0 [CI, 3.0–5.2]; P < 0.001 | ||||
Cole et al. (2000) | N = 5,234 | HR recovery | Age, gender, chronotropic response to exercise, habitual exercise, smoking, resting blood pressure, resting HR, cholesterol level, education, and income | Unadjusted: not stated |
Gender not reported | Adjusted: 2.58 [CI, 2.06–3.20] | |||
Nishime et al. (2000) | N = 9,454; 77 % men | HR recovery | Resting systolic blood pressure considered as a continuous variable, body mass index; use of non dihydropyridine calcium channel blockers, and lipid-lowering drugs, diabetes, insulin use, known hypercholesterolemia, documentation of total cholesterol value, known prior coronary heart disease, prior myocardial infarction, prior coronary artery bypass graft surgery, reason for test (screening or not), and presence of chronic obstructive pulmonary disease | Unadjusted: 4.16 [CI, 3.33–5.19]; p < 0.001 |
Adjusted: 2.13 [CI, 1.63–2.78]; p < 0.001 | ||||
Shetler et al. (2001) | 2,193 men with previous MI | HR recovery | Unadjusted: 2.6 [CI, 2.4–2.8] | |
Adjusted: not stated | ||||
Kleiger et al. (1987) | N = 808; gender not reported | HRV | Average of normal RR intervals, ventricular premature complex frequency, ventricular pairs or runs | Unadjusted: 2.7 [CI not reported] |
Adjusted: 5.3 [CI not reported] | ||||
Tsuji et al. (1994) | N = 736; 40 % men | HRV | Age, sex, history of myocardial infarction or congestive heart failure, presence of complex or frequent ventricular premature beats, and diuretic use | Unadjusted: 1.87 [CI, 1.55–2.26]; p = 0.0001 |
Adjusted: 1.70 [CI, 1.37–2.09]; p = 0.0001 | ||||
Gerritsen et al. (2001) | N = 605; gender not reported | HRV | Models for subjects with diabetes were adjusted for age, gender, and known diabetes; high-risk subjects were adjusted for age and gender | Unadjusted: 1.69 [CI, 1.02–2.80] |
Adjusted: 2.25 [CI, 1.13–4.45] | ||||
Liao et al. (2002) | N = 11,654; 42 % men | HRV | Baseline age, sex, ethnicity-centre, cigarette smoking status, and mean heart rate | Unadjusted: not stated |
Adjusted: 2.03 [CI, 1.28–3.23], 1.60 [CI, 1.12–2.27], 1.50 [CI, 0.65–3.42], and 1.27 [CI, 0.84–1.91] for incident MI, incident CHD, fatal CHD, and non-CHD deaths, respectively, comparing lowest quartile to the upper most three quartiles of HF | ||||
La Rovere et al. (1998) | N = 1,284 total; 87 % men | HRV | LVEP and VPC | Unadjusted: 5.3 [2.49–11.4]; p < 0.0001 |
Adjusted: 3.2 [CI, 1.42–7.36]; p = 0.005 | ||||
Camm et al. (2004) | 3,717 total; 78 % men | HRV | Age, LVEF, NYHA class, sex, diabetes, beta-blocker use at baseline | Unadjusted: not started |
Adjusted: 1.46 [CI, 1.10–1.94]; p = 0.009 |
Table 2.2
Studies of vagal function and cardiovascular risk factors
Risk factorsfor CVD | Studies | Subject and sample size | Measures employed | Controlled variables | Relative risk |
|---|---|---|---|---|---|
Hypertension | Liao et al. (1996) | N = 2,061; 45 % men | HRV and hypertension | Age, race, gender, current smoking, diabetes, and education | Unadjusted: not stated |
Adjusted: 1.00, 1.46 [CI, 0.61–3.46], 1.50 [CI, 0.65–3.50] and 2.44 [CI, 1.15–5.20] from the highest to the lowest quartile of HF | |||||
Hypertension | Singh et al. (1998) | N = 2,042; 46 % men | HRV and hypertension | Age, BMI, smoking, and alcohol consumption | Unadjusted: not stated |
Adjusted: LF power: Men 1.38 [CI, 1.04–1.83] p < 0.05; Women 1.12 [CI, 0.86–1.46] p = ns | |||||
Hypertension | Schroeder et al. (2003) | n = 11,061; men and women | HRV, hypertension and blood pressure | Age, sex, race, study centre, diabetes, smoking, education, and BMI | Unadjusted: not stated |
Adjusted: SDNN: 1.24 [CI, 1.10–1.40]; RMSSD: 1.36 [CI, 1.21–1.54]; RR interval 1.44 [CI, 1.27–1.63] | |||||
Diabetes | Liao et al. (1995) | n = 1,933; 44 % men | HRV and fasting glucose | Age, race, and gender | Unadjusted: not stated |
Adjusted: mean HF: diabetics: 0.78, non-diabetics: 1.27, p < 0.01 Adjusted among non-diabetics: inverse relationship between serum insulin and HF lowest quartile to highest quartile of insulin: 1.34 and 1.14, respectively | |||||
Diabetes | Singh et al. (2000) | n = 1,918; 57 % men | HRV and blood glucose levels | Age, sex, heart rate, body mass index, antihypertensive and cardiac medications, systolic and diastolic blood pressures, smoking, and alcohol and coffee consumption | Unadjusted: r = −0.21±; p < 0.05 |
Adjusted: r = −.05±; p < 0.0001 | |||||
Diabetes | Carnethon et al. (2003) | n = 8,185; gender not reported | Autonomic dysfunction (high HR and low HRV) and development of type diabetes | Age, race, sex, study centre, education, alcohol drinking, current smoking, prevalent coronary heart disease, physical activity, and BMI | Unadjusted: not stated |
Adjusted: LF lowest to highest quartile 1.2 [CI, 1.0–1.4] | |||||
Diabetes | Panzer et al. (2002) | n = 5,190; 61 % men | Fasting glucose and HR recovery | Age, gender, BMI, resting blood pressure, antihypertensive treatment, cholesterol, education, and alcohol consumption | Unadjusted: not stated |
Adjusted: impaired HR recovery more common in diabetics RR = 1.61 [CI, 1.35–1.92] and in those with impaired fasting glucose RR = 1.34 [CI, 1.2–1.5] | |||||
Cholesterol | Christensen et al. (1999) | 47 men with heart disease and 38 healthy men | Vagal tone and cholesterol | Plasma lipids, lipoproteins, age and BMI | Unadjusted: inverse correlation between total cholesterol and LDL, respectively. Healthy men: r = −0.38, p < 0.05 and r = −0.22, ns; 1.34 [CI, 1.20–1.50]; Men with IHD: r = −0.38, p < 0.05 and r = −0.37, p < 0.05 |
Adjusted: Men with IHD: inverse correlation between total cholesterol and SDNN: r = −0.43, p < 0.01 Adjusted: Healthy men: inverse correlation between total cholesterol and SDNN: r = −0.28, p < 0.05 | |||||
Cholesterol | Kupari et al. (1993) | n = 88; 47 % men | Short-term HRV and cholesterol | Physical activity, smoking and alcohol consumption | Unadjusted: not stated |
Adjusted: inverse correlation between RMSSD and LDL [β = −0.22; p = 0.008]; and total spectral power and LDL [β = −0.25; p = 0.007] | |||||
Cholesterol | Wannamethee and Shaper (1994) | n = 5,597; 100 % men | HR and cholesterol | Age, BMI, smoking, physical activity, alcohol consumption, social class and FEV1 | Unadjusted: correlations between HR and triglyceride levels (r = 0.15, p < 0.0001); cholesterol (r = 0.07, p < 0.0001); HDL cholesterol (r = −0.04; p < 0.01) |
Adjusted: correlations remained significant between HR and Triglycerides p < 0.05; cholesterol p < 0.05 | |||||
Cholesterol | Bonaa and Arnesen (1992) | n = 19,152; 51 % men | HR and cholesterol | Age, BMI, square of BMI, height, physical activity, cigarettes, and coffee | Unadjusted: not stated |
Adjusted: HR > 89 bpm vs. HR < 60 bpm for non-HDL: Men = 14.5 % higher non-HDL; Women = 12.5 % higher non-HDL; For triglycerides: Men = 36.3 % higher; Women = 22.2 % higher | |||||
Smoking | Yotsukura et al. (1998) | n = 20; 100 % men | Vagal tone and smoking | Within-subject design | HRV increased after smoking cessation: with withdrawal syndrome (Pre vs. Post): LF = 31.9 ± 7 and 39.4 ± 9.6 years; HF = 18.0 ± 6.0 and 23.7 ± 6.8 years; RMSSD = 40.8 ± 13.3 and 46.0 ± 18.2 years; without withdrawal syndrome: LF = 30.5 ± 4.9 and 36.3 ± 5.0*; HF = 17.3 ± 6.0 and 19.8 ± 2.9; RMSSD = 40.0 ± 14.4 and 46.5 ± 12.9; *p < 0.05 vs. smoking; ⃰p < 0.01 vs. smoking |
Smoking | Nabors-Oberg et al. (2002) | n = 16; 50 % men | HRV and smoking | Within-subject design | HF: Baseline = 896 (1,346) vs. Cigarette 1 = 338 (687), p < 0.05 |
Smoking | Hayano et al. (1990) | Study I: n = 9; 100 % men; Study II: n = 81; 100 % men | Vagal tone and smoking | Within-subject design | Study I: HF decreased after 3 min of smoking (p = 0.0061); Study II: ≤30 years: HF lower in heavy smokers compared to moderate and non-smokers; >30 no difference in older group among the three smoking groups |
Smoking | Minami et al. (1999) | n = 42; 100 % men | Vagal tone and smoking cessation | Within-subject design | Smoking period vs. nonsmoking period: LF = 5.28 ± 0.11 vs. 5.76 ± 0.11, p < 0.0001; HF = 4.37 ± 0.17 vs. 5.00 ± 0.16; p < 0.0001 |
Physical inactivity | Rossy and Thayer (1998) | n = 40; 52 % men | Vagal tone and habitual exercise | BMI | Positive relationship between indices of vagal tone and fitness: HP; (t (36) = 2.25, p = 0.015, rpb = 0.35), % BB50 (t (36) = 3.02, p = 0.0025, rpb = 0.45), SD (t (36) = 1.78, p = 0.04, rpb = 0.28), MSD (t (36) = 1.93, p = 0.03, rpb = 0.31), and |
HF (t (36) = 1.80, p = 0.04, rpb = 0.29) | |||||
Physical inactivity | Rennie et al. (2003) | n = 3,328; 70 % men | Physical activity and vagal tone or HR | Smoking and high alcohol intake | Unadjusted: not stated |
Adjusted: LF Men; [284.6, 332.0, 337.0, 342.4] p < 0.01; LF Women; [233.5, 246.9, 233.5, 243.2, 0.88]; p = 0.88; HF Men; [104.8, 118.3, 116.3, 125.2] p < 0.05; HF Women; [133.8, 146.9, 129.8, 141.0] p = 0.61; for total physical activity quartile low through high | |||||
Obesity | Petretta et al. (1995)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|