Elective Endovascular Repair of Abdominal Aortic Aneurysm
REID A. RAVIN and PETER L. FARIES
Presentation
The patient is a 77-year-old man who was discovered to have an abdominal aortic aneurysm (AAA) by ultrasound performed for evaluation of intermittent right upper quadrant discomfort. The ultrasound demonstrated no cholelithiasis, thickening of the gallbladder wall, or dilatation of the common bile duct and was not remarkable for findings other than an AAA. A subsequent computed tomography (CT) scan is ordered. The CTA confirms the presence of a 7.9 cm AAA (Figure 1).
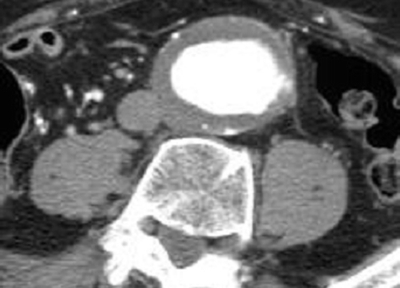
FIGURE 1 Cross sectional image showing large AAA.
Differential Diagnosis
The diagnosis of an asymptomatic AAA has been established using radiographic studies. There is no evidence of rupture or acute expansion. Further decisions regarding treatment must now incorporate evaluation of the patient’s overall condition, ability to tolerate surgery, life expectancy, and likelihood of aneurysm rupture. Given the large size of the aneurysm, rupture within 12 months is likely. Intervention is therefore indicated for the prevention of rupture, if the patient does not have a terminal illness and if he is able to tolerate surgical repair.
Workup
The patient’s ability to tolerate conventional open aneurysm repair should be evaluated. History and physical examination should focus on assessing functional status, and identifying factors that may complicate a surgical approach, as well as any comorbid conditions that may affect a patient’s long-term prognosis or perioperative morbidity and mortality. Particular attention should be directed at evaluating a patient’s cardiac and pulmonary status as these diseases are commonly comorbid with AAA. Renal function should also be assessed. According to AHA guidelines, patients with multiple risk factors and poor functional capacity who require vascular surgery should undergo noninvasive stress testing prior to surgery. For patients with a known pulmonary condition or in whom there is suspicion of undiagnosed pulmonary disease, pulmonary function testing is justified.
Case Continued
Upon obtaining additional history, it is determined that the patient requires elevation of his head on two pillows to sleep at night, he cannot ascend a flight of stairs due to the onset of significant dyspnea, and he awakens two to three times a night to urinate. He has a 45 pack-year history of smoking but discontinued smoking 10 years ago. Physical examination demonstrates an 8-cm pulsatile abdominal mass that is nontender. He has normal femoral pulses bilaterally. His colostomy site from his prior abdominoperineal resection is intact and functioning well, and his prior abdominal surgery did not significantly limit his ambulation. He has 2+ pedal edema without ulceration or evidence of venous insufficiency. Further cardiac and pulmonary evaluation is ordered.
Report of Diagnostic Tests
An echocardiogram and adenosine thallium stress test are performed. The patient is demonstrated to have global ventricular hypokinesis with a markedly reduced ejection fraction of 35%. There are fixed myocardial perfusion defects in the left anterior and lateral wall regions, but no reversible perfusion defects.
Pulmonary function tests indicate significantly reduced pulmonary capacity and functional reserve with forced expiratory volume in 1 second (FEV1) of 0.9 L.
Diagnosis and Treatment
Several factors suggest that this patient will be at significantly increased risk for morbidity and mortality if conventional AAA repair is performed. His cardiac and pulmonary capacity is considerably reduced. In addition, the presence of a stoma and the loss of abdominal domain make his a “hostile” abdomen and increase the difficulty of conventional repair and increased potential for prosthetic graft infection. Therefore, this patient should be considered for endovascular aneurysm repair (EVAR) using a stent graft.
Anatomic evaluation is performed to determine if a stent graft will be successful in excluding the aneurysm from the arterial circulation, thereby preventing aneurysm rupture. To achieve aneurysm exclusion, undilated arterial areas must be present proximal and distal to the aneurysm. These fixation zones in the immediate infrarenal aorta proximally and common iliac arteries distally allow for sealing of the aneurysm and prevent arterial flow into the aneurysm sac. An undilated aortic segment at least 10 mm in length should be present distal to the renal arteries for successful repair. Excessive angulation of the pararenal aorta may also prevent adequate stent graft fixation and sealing. A distal region of nonaneurysmal artery must also be present to achieve fixation and sealing. Typically, this occurs in the common iliac arteries; however, stent grafts may be extended into the external iliac if the common iliac is dilated. Extension into the external iliac arteries requires occlusion of the internal iliac arteries, which may be associated with ischemic complications of the pelvis and gluteal region. Preoperative embolization of the internal iliac arteries prior to EVAR is being done with increasing frequency and is associated with a significant incidence of postoperative buttock claudication, as well as rare, but serious ischemic complications to the lower gastrointestinal tract. Bypass to the internal iliac arteries at the time of EVAR can be performed if it is essential to preserve arterial perfusion. Alternatively, newer branched iliac devices are currently being evaluated for use in aneurysms involving the iliac bifurcation. Finally, the vessels used to access the aorta must also be of an adequate caliber to allow passage of the endovascular stent graft. Typically, this is carried out from the femoral arteries through the external iliac arteries.
The current patient undergoes a contrast-enhanced spiral CT scan with images obtained at 2.5-mm intervals to allow measurement of the proximal aortic and common iliac artery diameters. Three-dimensional reconstruction and center line calculations obtained from the CT scan can be used to determine the length of the arterial segment that is to be excluded by the stent graft. That is the distance from the distal-most renal artery to the bifurcation of the common iliac arteries bilaterally. These measurements are used to determine the length of the stent graft.
CT Scan and Angiogram
CT Scan
The proximal aortic fixation zone is determined to be 25 mm in diameter and 18 mm long. The common iliac arteries are 14 mm in diameter, and the distance from the renal ostia to the iliac bifurcations is 147 mm on the right and 152 mm on the left.
Case Continued
The most appropriate course of management for this patient is endovascular AAA repair. His anatomy will allow successful exclusion of the aneurysm by placement of a stent graft. He exhibits multiple factors that would place him at increased risk for conventional repair. He has a large AAA and no terminal conditions, so he is likely to die from aneurysm rupture if repair is not performed.
Surgical Approach
Repair is performed in the operating room on a radiolucent table. The patient may receive local, epidural, or general anesthesia. Because he exhibits significant pulmonary disease, regional anesthesia without the requirement for intubation is preferable. The abdomen and legs are prepared in a sterile manner in the event that conversion to open repair becomes necessary. The stoma site is excluded from the sterile field. Exposure of the common femoral arteries is performed bilaterally, and an angiographic guidewire is introduced into the abdominal aorta. Alternatively, percutaneous access can be obtained to the femoral arteries using the preclose technique, wherein two perclose devices (Proglide, Abbot Vascular, Redwood City, CA) are deployed in the femoral arteries prior to advancing large sheaths and/or delivery catheters. The perclose devices, which contain sutures and preformed slip knots, are typically deployed medially and laterally at 45-degree angles. The sutures are then tagged and placed aside for the duration of the intervention, and the artery is closed at the end of case by pushing down the preformed knots on to the artery after the delivery devices/catheters are removed.
After access is obtained using percutaneous access or femoral cut down, a flush catheter is placed in the aorta over the wire to allow the performance of angiography. A stiff guidewire is placed through a selective angiographic catheter from the ipsilateral femoral artery. After administration of systemic anticoagulation, the main body of the stent graft is brought into position in the aorta over the stiff guidewire and deployed in the infrarenal aorta. The short contralateral gate of the main body of the stent graft is then cannulated from the contralateral femoral access site using a guidewire. A pigtail catheter is tracked over the guidewire and spun within the stent graft to confirm intraluminal position. A second stiff guidewire is then brought through the short contralateral gate, and the contralateral iliac delivery system is tracked into position through the contralateral femoral arteriotomy site. After deployment of the contralateral iliac limb, completion angiography is performed to ensure complete exclusion of the aneurysm Table 1.
TABLE 1. Elective Endovascular Repair of AAA