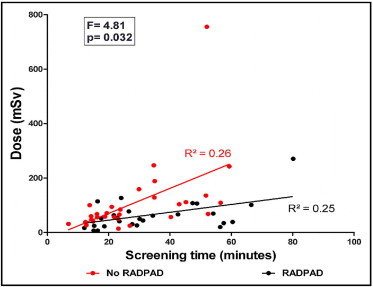
The RADPAD is a lead-free surgical drape containing bismuth and barium that has been demonstrated to reduce scatter radiation exposure to primary operators during fluoroscopic procedures. It is not known to what degree the RADPAD reduces radiation exposure in operators who perform highly complex percutaneous coronary intervention (PCI) requiring prolonged fluoroscopic screening times. Sixty consecutive patients due to undergo elective complex PCI involving rotational atherectomy, multivessel PCI, or chronic total occlusions were randomized in a 1:1 pattern to have their procedures performed with and without the RADPAD drape in situ. Dosimetry was performed on the left arm of the primary operator. There were 40 cases of chronic total occlusion, including 28 with contralateral injections; 15 cases involving rotational atherectomy; and 5 cases of multivessel PCI. There was no significant difference in screening times or dose-area products between the 2 patient groups. Primary operator radiation dose relative to screening time (RADPAD: slope = 1.44, R 2 = 0.25; no RADPAD: slope = 4.60, R 2 = 0.26; analysis of covariance F = 4.81, p = 0.032) and dose-area product (RADPAD: slope = 0.003, R 2 = 0.26; no RADPAD: slope = 0.011, R 2 = 0.52; analysis of covariance F = 12.54, p = 0.008) was significantly smaller in the RADPAD cohort compared to the no-RADPAD group. In conclusion, the RADPAD significantly reduces radiation exposure to primary operators during prolonged, complex PCI cases.
Ionizing radiation remains an integral part of percutaneous coronary intervention (PCI). For staff members employed in a cardiac catheterization suite, chronic exposure to low-dose radiation confers a small but stochastic risk for inducing malignant disease, skin damage, or eye problems. In any given PCI procedure, the amount of radiation is determined by the operator, and significant variations can be observed among different operators. However, certain procedural factors are associated with increased radiation doses. Longer procedures involving rotational atherectomy, chronic total occlusions (CTOs), bifurcation lesions, and multivessel PCI often result in higher radiation exposure. The RADPAD (Worldwide Innovations & Technologies, Inc., Kansas City, Kansas) is a sterile surgical drape containing radiation protection materials (bismuth and barium). Placed appropriately on the patient between the image intensifier and the operator, the RADPAD will reduce scatter radiation. It has been shown to reduce radiation exposure in routine PCI procedures, cardiac resynchronization therapy device implantation, and fluoroscopically guided electrophysiologic procedures. We sought to assess the efficacy of RADPAD drapes in reducing radiation dose experienced by operators during prolonged, complex PCI procedures.
Methods
Sixty consecutive patients due to undergo elective complex PCI were identified. Cases with potentially long screening times (STs) were selected, comprising procedures involving multivessel (≥2) PCI, PCI requiring rotational atherectomy, or complex CTOs. Patients were randomly assigned to undergo the procedure with or without the RADPAD in situ, with 30 patients in each group.
The PCI procedures were carried out by 2 interventional cardiologists with backgrounds in performing high-volume complex PCI procedures. During the PCI procedures, standard shielding equipment was used, including a lead coat, thyroid shield, and protective lenses, in addition to a lead shield suspended from the ceiling between the image intensifier and operator. Both operators had undergone radiation awareness courses and were familiar with the optimal placement of the RADPAD drape.
For cases involving right radial arterial access, the RADPAD was positioned superior and medial to the sheath insertion point, immediately below the lead shield suspended from the ceiling between the image intensifier and operator. For cases involving femoral arterial access, the RADPAD was positioned superior to the sheath insertion point, immediately below the lead shield suspended from the ceiling between the image intensifier and operator.
To standardize the study in every case, the dosimeter was placed on the upper, outer aspect of the left arm of the first operator, at the level of the mid humerus. Dosimetric measurements were obtained using an Unfors EDD meter (Unfors Instruments AB, Billdal, Sweden). The dosimeter was commenced at the start of the procedure, and the dose was recorded immediately after the end of the PCI. STs and dose-area products (DAPs) for each individual procedure were collated prospectively. Student’s independent t test was used to compare mean values of different parameters of radiation exposure. Scatterplot analysis and linear regression slopes of radiation dose relative to ST and DAP were performed. Comparison between the slopes was performed using analysis of covariance to assess for statistical significance. A p value <0.05 was accepted as statistically significant.
Results
Data were collated successfully from 60 patients. Most cases involved men (49 of 60), and the mean age of the study group was 68.9 ± 9.2 years.
There were 40 cases involving CTO, of which 28 used contralateral injections (angiography of contralateral coronary artery to opacify the distal portion of CTO vessel) and 9 involved a retrograde approach (PCI performed by passing the coronary wire through a distal CTO cap via collateral circulation). There were 15 cases of rotational atherectomy and 5 cases of multivessel PCI. The relative types of PCI procedure performed were similar in the 2 groups. In 6 cases, PCI was performed via the femoral artery, and the remaining procedures were performed via the radial artery ( Table 1 ).
| Variable | RADPAD | p Value | |
|---|---|---|---|
| Yes (n = 30) | No (n = 30) | ||
| Men/women | 25/5 | 24/6 | |
| Age (years) | 68.7 ± 8.3 | 69.1 ± 10.1 | 0.87 |
| Access Site | |||
| Radial | 14 | 18 | |
| Femoral/femoral | 3 | 3 | |
| Radial/femoral | 12 | 8 | |
| Radial/radial | 1 | 1 | |
| PCI type | |||
| Rotational atherectomy | 6 | 9 | |
| CTO | 21 | 19 | |
| Multivessel | 3 | 2 | |
| ST (minutes) | 33.4 (12.1–80.2) | 28.6 (6.9–59.3) | 0.28 |
| DAP (Gy · cm 2 ) | 13,182 (3,538–40,705) | 11,692 (1,684–36,546) | 0.51 |
| Dose (mSv) | 64.8 (6.9–270.6) | 109.9 (14.2–755.5) | 0.09 |
| Dose/DAP (mSv/[Gy − cm 2 ] | 0.0063 | 0.0122 | 0.02 |
| Dose/ST (mSv/min) | 2.12 | 3.80 | 0.002 |
Overall, the cases were prolonged, with a mean ST of 31.0 ± 16.6 minutes and a mean DAP of 12,437 ± 8,656 Gy · cm 2 . There was a numeric trend toward the RADPAD cohort having longer STs and higher DAPs during their procedures than the no-RADPAD cohort, but this was not statistically significant ( Table 1 ). Conversely, the recorded mean radiation dose was numerically smaller in the RADPAD group than in the no-RADPAD group, and this trended toward, but did not reach, statistical significance ( Table 1 ). However, when the radiation dose was compared between the 2 groups relative to ST and DAP, the difference between the 2 groups became statistically significant. Comparing dose relative to ST, the RADPAD group received a significantly smaller dose of radiation compared to the no-RADPAD cohort (RADPAD: slope = 1.44; no RADPAD: slope = 4.60; analysis of covariance F = 4.81, p = 0.032; Figure 1 ) . When comparing the dose relative to the DAP used during each individual procedure, there was a significant difference between the 2 groups, with the RADPAD cohort receiving a smaller dose compared to the no-RADPAD group (RADPAD: slope = 0.003; no RADPAD: slope = 0.011; analysis of covariance F = 12.54, p = 0.008; Figure 2 ) .