A post hoc analysis of Systolic Heart failure treatment with the I f inhibitor ivabradine Trial (SHIFT) explored the efficacy and safety of ivabradine in severe heart failure (HF) as denoted by left ventricular ejection fraction (LVEF) ≤20% and/or New York Heart Association (NYHA) class IV. The SHIFT population (LVEF ≤35%, heart rate ≥70 beats/min, and sinus rhythm) comprised 712 patients with severe (defined previously) and 5,973 with less severe (NYHA classes II or III and LVEF >20%) HF, all randomized to ivabradine or placebo on a background of guideline-defined standard care. The rate of primary composite end point of cardiovascular death or HF hospitalization with placebo was higher in severe (42%) than less severe (27%) HF (p <0.001). Treatment with ivabradine in severe HF was associated with relative risk reductions indistinguishable from those of less severe disease for the primary end point (16% reduction), all-cause death (22%), cardiovascular death (22%), HF death (37%), and HF hospitalization (17%; all p values for interaction: NS). NYHA class improved in 38% (n = 129) ivabradine-treated patients with severe HF versus 29% (n = 104) placebo-treated patients (p = 0.009). In the 272 patients with severe HF and baseline heart rate ≥75 beats/min (the indication approved by the European Medicines Agency), ivabradine reduced the primary end point by 25% (p = 0.045), HF hospitalization by 30% (p = 0.042), and cardiovascular death by 32% (p = 0.034). Ivabradine’s safety profile in severe HF was indistinguishable from less severe. In conclusion, our analysis confirms that heart rate reduction with ivabradine can be safely used in severe HF and may improve clinical outcomes independently of disease severity.
The clinical severity of congestive heart failure (HF) is commonly defined in terms of New York Heart Association (NYHA) functional class. Severe HF, that is, NYHA class IV, is associated with relatively poor outcomes (28% mortality rate at 1 year vs 7% and 15% for NYHA classes II and III, respectively). Some studies indicate an even poorer prognosis: trial data suggested that 56% of patients in NYHA class IV die from worsening HF within 1 year. Severity of HF can also be understood in terms of left ventricular systolic function: trial data indicate a 39% increase in all-cause mortality rate for each 10% decrement in left ventricular ejection fraction (LVEF) below normal; most HF-related deaths occur in patients with the lowest LVEF (defined as ≤22%). Patients with severe HF are the most difficult to treat and are also the most in need of better-than-conventional treatment for HF (careful optimization of treatment may not be sufficiently effective ). The Systolic Heart failure treatment with the I f inhibitor ivabradine Trial (SHIFT) study demonstrated that heart rate reduction with the I f inhibitor, ivabradine, significantly improved clinical outcomes in patients with chronic systolic HF. This post hoc analysis of SHIFT explores the efficacy and safety of ivabradine in patients with severe HF (NYHA class IV and/or LVEF ≤20%).
Methods
The trial design, procedures, and the main results of the SHIFT study have been described in detail elsewhere. Briefly, this event-driven, multicenter, randomized, double-blind, placebo-controlled trial included patients with moderate-to-severe HF and left ventricular systolic dysfunction. Participants were in sinus rhythm and had a heart rate ≥70 beats/min (12-lead electrocardiography) and LVEF ≤35%. Eligible patients had stable symptomatic HF (≥4 weeks) but had been hospitalized for worsening HF in the 12 months before inclusion. All patients were receiving stable background therapy according to the 2008 European guidelines. Investigators were encouraged to maintain participants as closely as possible to target dosages, notably with β blockade. Patients were randomly allocated to ivabradine or placebo at a starting dose of 5 mg twice daily; doses were adjusted upward or downward (2.5, 5, or 7.5 mg twice daily) at every visit according to heart rate at rest and tolerability. The SHIFT study conformed to the Declaration of Helsinki (1964 and its text revisions), and ethics committee approval was obtained in all involved countries. All subjects gave written informed consent to participate in the study.
The primary outcome was the composite of cardiovascular death or hospitalization for worsening HF. Other end points included the individual components of the primary end point, all-cause death, HF death, and hospitalization for any cause. Outcomes were analyzed on a time-to-first event basis, and all end points were adjudicated by a blinded end point validation committee. NYHA class was recorded at baseline and at every 4-monthly visit throughout the trial. The median follow-up was 22·9 months (interquartile range 18 to 28).
In this post hoc analysis, we assessed the effect of ivabradine on outcomes according to the severity of HF at baseline. Patients with severe HF were defined as all patients in NYHA class IV and NYHA classes II or III with LVEF ≤20%. The complementary group of patients with less severe HF were those in classes II or III with LVEF >20%. To test the relevance of the results in patients with severe HF according to this definition, the following subgroups were also analyzed: patients in NYHA class IV; patients in NYHA classes II or III with LVEF ≤20%; patients in NYHA classes II or III with LVEF ≤15%; and patients in NYHA class IV with LVEF ≤15%. The current indication for ivabradine according to the European Medicines Agency includes heart rate at rest ≥75 beats/min. Therefore, we also assessed ivabradine’s effect in patients with severe or less severe HF and heart rate at rest ≥75 beats/min at baseline. In a separate analysis, we explored the impact of treatment on differences in change in functional capacity (NYHA class) over the study period according to the severity of HF at baseline.
Baseline characteristics are presented as mean ± SD for continuous variables and numbers of patients (percentages) for categorical variables. The characteristics of the patients with severe and less severe HF at baseline were compared using a 2-sample t test for continuous variables and a chi-square test for categorical variables. To study the effect of ivabradine on the relation between HF severity and outcome, treatment effects were estimated using a Cox proportional hazards model, adjusted for β-blocker use, to produce hazard ratios with 95% confidence intervals and p values for each HF severity group. A Cox proportional hazards model adjusted for prognostic factors at baseline (β-blocker intake, heart rate, NYHA class, LVEF, ischemic cause of HF, age, systolic blood pressure, and creatinine clearance) was also used to confirm the trends observed with the other model. Evidence of a trend in the estimated treatment effect across groups was tested by adding a factorial interaction between treatment group and HF severity to a model containing HF severity and treatment group. Change in NYHA class over the study period is presented as numbers of patients (percentages) improving by at least 1 NYHA class, with between-group differences (p values) estimated using a chi-square test. Adverse events and adverse events leading to withdrawal are presented as numbers of patients (percentages) for those receiving ivabradine and placebo in patients with at least 1 intake of study treatment. Between-group differences (p values) in adverse events were estimated with a chi-square or Fisher’s exact test, as appropriate. All analyses were carried out by the independent statistical center at the Robertson Centre for Biostatistics (Glasgow, United Kingdom), using SAS software (version 9.2; SAS Institute Inc., Cary, North Carolina).
Results
Of the 6,505 patients randomized in SHIFT, 712 had severe HF, that is, NYHA class IV HF and/or LVEF ≤20%, versus 5,793 with less severe HF. Of these 712 patients, 343 (48%) received ivabradine; of the total cohort of 6,505 patients, 2,898 patients (50%) were allocated to ivabradine. Of the 712 patients with severe HF, 111 were in NYHA class IV and 601 were in NYHA classes II or III but had LVEF ≤20%.
The baseline characteristics of the patients with severe or less severe HF are presented in Table 1 . Consistent with their disease status, patients with severe HF had markedly lower LVEF (19.6% vs 30.2% in patients with less severe HF), lower body mass index and blood pressure, longer time since diagnosis of chronic HF, and higher heart rate at rest and serum creatinine. Fewer patients with severe HF had hypertension, a history of myocardial infarction, or an ischemic cause of HF. Patients with severe HF were also more likely to be taking diuretics but less likely to receive high doses of β blockers. Of the patients with severe HF, 513 (72%) had heart rate ≥75 beats/min, versus 3,637 (63%) of the patients with less severe HF. There were no substantial differences between the ivabradine and placebo subgroups in the severe and less severe HF groups, with the exception of rates of ischemic HF, previous myocardial infarction, diabetes, and β-blocker dose in the severe HF group, which indicates that the ivabradine subgroup had slightly more co-morbidities than the placebo group ( Table 1 ).
| Characteristic | Severe HF (n = 712) NYHA Class IV and/or LVEF ≤20% | Less Severe HF (n = 5,793) NYHA Class II or III and LVEF >20% | p Value | ||||
|---|---|---|---|---|---|---|---|
| All | Ivabradine (n = 343) | Placebo (n = 369) | All | Ivabradine (n = 2,898) | Placebo (n = 2,895) | ||
| Demographic characteristics | |||||||
| Age (yrs) | 60.1 ± 12.2 | 61.4 ± 11.8 | 59.0 ± 12.4 | 61.0 ± 11.3 | 61.2 ± 11.1 | 60.8 ± 11.4 | 0.057 |
| Men | 549 (77) | 269 (78) | 280 (76) | 4,421 (76) | 2,193 (76) | 2,228 (77) | 0.64 |
| Caucasian | 558 (78) | 273 (80) | 285 (77) | 5,213 (90) | 2,606 (90) | 2,607 (90) | <0.001 |
| Current smokers | 128 (18) | 67 (20) | 61 (17) | 990 (17) | 474 (16) | 516 (18) | 0.55 |
| Body mass index (kg/m 2 ) | 26.7 ± 4.8 | 26.4 ± 4.8 | 26.8 ± 4.8 | 28.2 ± 5.1 | 28.2 ± 5.1 | 28.1 ± 5.0 | <0.001 |
| Cardiac parameters | |||||||
| Heart rate at rest (beats/min) | 82.1 ± 10.7 | 81.7 ± 10.3 | 82.6 ± 11.0 | 79.6 ± 9.4 | 79.5 ± 9.4 | 79.8 ± 9.5 | <0.001 |
| Systolic blood pressure (mm Hg) | 115.8 ± 16.4 | 116.2 ± 16.7 | 115.5 ± 16.1 | 122.4 ± 15.8 | 122.6 ± 15.8 | 122.1 ± 15.7 | <0.001 |
| Diastolic blood pressure (mm Hg) | 72.6 ± 10.0 | 72.2 ± 9.8 | 72.9 ± 10.1 | 76.0 ± 9.4 | 76.2 ± 9.5 | 75.9 ± 9.2 | <0.001 |
| LVEF (%) | 19.6 ± 4.8 | 19.6 ± 4.7 | 19.6 ± 4.9 | 30.2 ± 3.8 | 30.2 ± 3.9 | 30.2 ± 3.8 | <0.001 |
| NYHA class III/IV vs class II | 442 (62) | 221 (64) | 221 (60) | 2,892 (50) | 1,434 (50) | 1,458 (50) | <0.001 |
| eGFR (ml/min/1.73 m 2 ) | 74.0 ± 25.9 | 72.5 ± 24.2 | 75.4 ± 27.4 | 74.6 ± 22.5 | 74.7 ± 22.7 | 74.6 ± 22.4 | 0.46 |
| Serum creatinine (μmol/L) | 99.2 ± 28.1 | 100.4 ± 28.0 | 98.1 ± 28.1 | 96.4 ± 26.2 | 96.2 ± 26.3 | 96.5 ± 26.1 | 0.007 |
| Medical history | |||||||
| Ischemic HF | 385 (54) | 204 (59) | 181 (49) | 4,033 (70) | 2,011 (69) | 2,022 (70) | <0.001 |
| Time since HF diagnosis (yrs) | 48.5 ± 58.4 | 48.9 ± 58.2 | 48.1 ± 58.6 | 41.1 ± 49.3 | 41.4 ± 49.9 | 40.9 ± 48.7 | <0.001 |
| Myocardial infarction | 324 (46) | 166 (48) | 158 (43) | 3,342 (58) | 1,663 (57) | 1,679 (58) | <0.001 |
| Hypertension | 365 (51) | 177 (52) | 188 (51) | 3,949 (68) | 1,985 (68) | 1,964 (68) | <0.001 |
| Diabetes | 203 (29) | 105 (31) | 98 (27) | 1,776 (31) | 868 (30) | 908 (31) | 0.24 |
| Chronic obstructive pulmonary disease | 105 (15) | 55 (16) | 50 (14) | 625 (11) | 303 (10) | 322 (11) | 0.002 |
| History of atrial fibrillation or flutter | 48 (7) | 21 (6) | 27 (7) | 474 (8) | 242 (8) | 232 (8) | 0.18 |
| Treatment at randomization | |||||||
| β Blocker | 620 (87) | 296 (86) | 324 (88) | 5,200 (90) | 2,601 (90) | 2,599 (90) | 0.028 |
| β-Blocker dose ∗ | |||||||
| ≥50% of target dose | 283 (46) | 126 (43) | 157 (49) | 2,898 (57) | 1,455 (57) | 1,433 (57) | <0.001 |
| Target dose | 136 (22) | 59 (20) | 77 (24) | 1,352 (27) | 684 (27) | 668 (26) | 0.021 |
| ACE inhibitor | 555 (78) | 270 (79) | 285 (77) | 4,561 (79) | 2,295 (79) | 2,266 (78) | 0.63 |
| Diuretic | 642 (90) | 310 (90) | 332 (90) | 4,772 (82) | 2,409 (83) | 2,363 (82) | <0.001 |
∗ Target dosages according to guidelines in the management of HF; percentages calculated for patients with relevant daily dose details.
During the trial, the mean dosage of ivabradine in severe and less severe HF groups was indistinguishable (6.5 ± 1.4 and 6.4 ± 1.4 mg twice daily, respectively; target dose 7.5 mg twice daily). Treatment with ivabradine was associated with similar reductions in heart rate at rest from baseline to 28 days in patients with severe (−15.2 ± 11.3 beats/min) and less severe HF (−15.4 ± 10.7 beats/min). The corresponding reductions in the placebo groups were −4.5 ± 11.2 and −4.6 ± 10.5 beats/min, respectively.
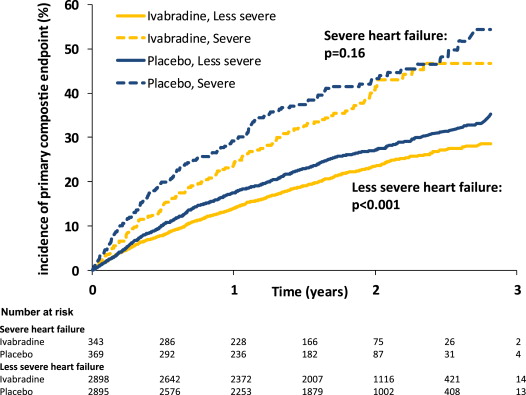
The event rates in patients with severe HF were systematically and substantially higher than those with less severe HF ( Table 2 ). For example, in the placebo group, the rate of primary composite end point was 42% for the patients with severe HF versus 27% for the less severe HF group (p <0.001; Figure 1 ). Similar differences were found for all other outcomes: all-cause death, cardiovascular death, HF death, hospitalization for worsening HF, and hospitalization for any cause. The effect of ivabradine failed to reach statistical significance versus placebo in patients with severe HF; nonetheless, there were substantial nominal reductions in relative risk for the primary composite end point (16%, p = 0.16), all-cause death (22%, p = 0.096), cardiovascular death (22%, p = 0.11), HF death (37%, p = 0.067), and hospitalization for worsening HF (17%, p = 0.21; Table 2 and Figure 1 ). However, in the group with severe HF and heart rate ≥75 beats/min, ivabradine was associated with significant reductions versus placebo in risk for the primary composite end point (25%, p = 0.045), all-cause death (34%, p = 0.018), cardiovascular death (32%, p = 0.034), HF death (59%, p = 0.005), and hospitalization for worsening HF (30%, p = 0.042). There was no evidence for a difference in the effect of treatment with ivabradine in the groups with severe and less severe HF in the whole population, or in those with heart rate ≥75 beats/min, for any of the outcomes examined (p >0.10 for interaction in all cases; Table 2 ). Analyses using a model adjusted for prognostic factors at baseline largely recapitulated these findings (data not shown).
| Outcome | Patients With Heart Rate ≥70 beats/min | Patients With Heart Rate ≥75 beats/min | ||||||
|---|---|---|---|---|---|---|---|---|
| Events, n (%) | HR (95% CI), ∗ p Value | p Value for Interaction | Events, n (%) | HR (95% CI), ∗ p Value | p Value for Interaction | |||
| Placebo | Ivabradine | Placebo | Ivabradine | |||||
| Primary composite end point † | ||||||||
| Less severe HF | 783 (27) | 669 (23) | 0.82 (0.74–0.91), <0.001 | 0.85 | 566 (31) | 459 (25) | 0.77 (0.68–0.87), <0.001 | 0.85 |
| Severe HF | 154 (42) | 124 (36) | 0.84 (0.67–1.07), 0.16 | 122 (45) | 86 (36) | 0.75 (0.57–0.99), 0.045 | ||
| All-cause death | ||||||||
| Less severe HF | 446 (15) | 425 (15) | 0.94 (0.82–1.07), 0.32 | 0.26 | 322 (18) | 288 (16) | 0.88 (0.75–1.03), 0.11 | 0.14 |
| Severe HF | 106 (29) | 78 (23) | 0.78 (0.58–1.05), 0.096 | 85 (31) | 52 (22) | 0.66 (0.47–0.93), 0.018 | ||
| Cardiovascular death | ||||||||
| Less severe HF | 393 (14) | 377 (13) | 0.94 (0.82–1.08), 0.41 | 0.26 | 286 (16) | 255 (14) | 0.88 (0.74–1.04), 0.12 | 0.21 |
| Severe HF | 98 (27) | 72 (21) | 0.78 (0.58–1.06), 0.11 | 78 (29) | 49 (20) | 0.68 (0.48–0.97), 0.034 | ||
| HF death | ||||||||
| Less severe HF | 108 (4) | 87 (3) | 0.79 (0.59–1.04), 0.096 | 0.43 | 90 (5) | 64 (4) | 0.69 (0.50–0.95), 0.023 | 0.14 |
| Severe HF | 43 (12) | 26 (8) | 0.63 (0.39–1.03), 0.067 | 36 (13) | 14 (6) | 0.41 (0.22–0.77), 0.005 | ||
| Hospitalization for worsening HF | ||||||||
| Less severe HF | 564 (19) | 428 (15) | 0.73 (0.64–0.83), <0.001 | 0.42 | 418 (23) | 307 (17) | 0.70 (0.60–0.81), <0.001 | 1.00 |
| Severe HF | 108 (29) | 86 (25) | 0.83 (0.63–1.11), 0.21 | 85 (31) | 56 (23) | 0.70 (0.50–0.99), 0.042 | ||
| Hospitalization for any cause | ||||||||
| Less severe HF | 1,182 (41) | 1,065 (37) | 0.87 (0.80–0.95), 0.001 | 0.21 | 796 (44) | 685 (38) | 0.82 (0.74–0.91), <0.001 | 0.77 |
| Severe HF | 174 (47) | 166 (48) | 1.01 (0.82–1.25), 0.92 | 136 (50) | 111 (46) | 0.85 (0.66–1.09), 0.21 | ||
∗ Adjusted for β-blocker use at randomization.
† Composite of cardiovascular death or hospitalization for worsening HF.