Aggressive low-density lipoprotein (LDL) cholesterol-lowering therapy is important for high-risk patients. However, sparse data exist on the impact of combined aggressive LDL cholesterol-lowering therapy in familial hypercholesterolemia (FH), particularly on side effects to changes in plasma coenzyme Q10 and proprotein convertase subtilisin/kexin type 9 levels. We enrolled 17 Japanese patients with heterozygous FH (12 men, 63.9 ± 7.4 years old) with single LDL receptor gene mutations in a prospective open randomized study. Permitted maximum doses of rosuvastatin (20 mg/day), ezetimibe (10 mg/day), and granulated colestimide (3.62 g/day) were introduced sequentially. Serum levels of LDL cholesterol decreased significantly by −66.4% (p <0.001) and 44% of participants achieved LDL cholesterol levels <100 mg/dl. There were no serious side effects or abnormal laboratory data that would have required the protocol to have been terminated except for 1 patient with myalgia. Coadministration of ezetimibe and granulated colestimide further lowered serum LDL cholesterol more than rosuvastatin alone without changing plasma coenzyme Q10 and proprotein convertase subtilisin/kexin type 9 levels. In conclusion, adequate introduction of this aggressive cholesterol-lowering regimen can improve the lipid profile of FH.
Familial hypercholesterolemia (FH) is the most severe monogenic hypercholesterolemia owing to a genetic defect or mutation of the low-density lipoprotein (LDL) receptor, apolipoprotein B, or proprotein convertase subtilisin/kexin type 9 (PCSK9) gene. PCSK9 binds to the epidermal growth factor-like repeat A domain of the LDL receptor, inducing LDL receptor degradation. Because FH is highly refractory to cholesterol-lowering medical therapy, monotherapy using a strong statin may not be enough to achieve the target LDL cholesterol level. Thus, a combination drug therapy with a different mechanism is needed. However, additional use of ezetimibe and/or resins may result in a depletion of other products downstream of the mevalonate pathway such as coenzyme Q10 (CoQ10) or inactivation of the sterol regulatory element binding protein 2 associated with the induction of PCSK9. We investigated the efficacy and safety of coadministration of maximum permitted doses of rosuvastatin, ezetimibe, and granulated colestimide in Japanese patients with heterozygous FH.
Methods
The study population consisted of 17 patients (12 men, mean ± SD 63.9 ± 7.4 years old) with heterozygous FH. All 17 subjects were heterozygous with a confirmed LDL receptor gene mutation and fulfilled our clinical diagnostic criteria for heterozygous FH: patients with primary hyper-LDL cholesterolemia (>160 mg/dl) with tendon xanthoma or those with first-degree relatives with previously diagnosed heterozygous FH showing primary hyper-LDL cholesterolemia (>160 mg/dl). Exclusion criteria of the present study were FH patients with a homozygous gene mutation, patients under LDL apheresis therapy or any immunomodulatory medication, patients with fasting serum triglyceride levels >500 mg/dl, patients with hepatic disease, or patients within 12 weeks after the onset of an acute myocardial infarction or stroke. Written informed consent to participate in the present study was obtained from each patient before entry in the study. Ethical committees of Kanazawa University Hospital and KKR Hokuriku Hospital approved the study protocol.
This study was conducted as a prospective open randomized study to investigate the efficacy and safety of coadministration of rosuvastatin (20 mg/day), ezetimibe (10 mg/day), and granulated colestimide (3.62 g/day) at the maximum doses permitted in Japan. All patients were outpatients at the beginning of the study. Any lipid-lowering agents had been washed out ≥4 weeks before entry in the present study. All study patients were placed on the following diet therapy: 25 to 30 kcal for ideal body weight, fat restriction <20% of total oral intake, cholesterol restriction <200 mg/day, and saturated fatty acid restriction <30% of total fatty acid.
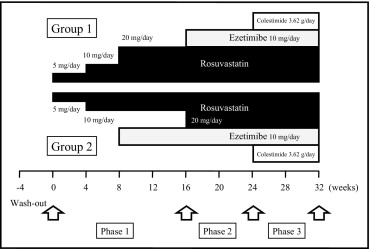
Study subjects were divided into 2 groups by an envelope method to elucidate the secondary end point of the present study: rosuvastatin 20 mg/day (group 1) versus rosuvastatin 10 mg/day coadministered with ezetimibe 10 mg/day (group 2) ( Figure 1 ) . All participants were started on a 4-week treatment with rosuvastatin 5 mg/day followed by another 4-week treatment of rosuvastatin 10 mg/day. The dose of rosuvastatin in group 1 was increased to 20 mg with an 8-week follow-up, whereas group 2 received ezetimibe 10 mg/day with an 8-week follow-up (phase 1). After phase 1, group 1 received ezetimibe 10 mg/day added to rosuvastatin for 8 weeks, whereas in group 2 the doses of rosuvastatin were increased to 20 mg with an 8-week follow-up (phase 2). In phase 3, groups 1 and 2 were given granulated colestimide 3.62 g (2 times/day before meals, 1 time in the morning and 1 time in the evening) added to the phase 2 treatment regimen. Because all but 1 participant were exposed to the same lipid-lowering drugs for >16 weeks during phases 2 and 3 and the end points were objective laboratory data, the influence of different treatments during phase 1 on the primary end points between groups 1 and 2 were likely to be minimal. Thus, all serum lipid, apolipoprotein, hepatic, muscular, CoQ10, and PCSK9 parameters from the 2 groups at baseline and phase 3 were combined.
Blood samples were obtained after an overnight fast after the wash-out period and phases 1, 2, and 3. Serum cholesterol and triglycerides were measured by an enzymatic method and high-density lipoprotein (HDL) cholesterol levels were measured by a polyamine–polymer/detergent method (Daiichi, Tokyo, Japan). Serum LDL cholesterol levels were calculated using the Friedewald formula. Apolipoproteins A-I, A-II, B, C-II, C-III, and E were determined as described previously. Plasma levels of CoQ10 were determined by a high-performance liquid chromatographic method as described previously. Plasma PCSK9 concentrations were determined using an enzyme-linked immunosorbent assay kit targeting human PCSK9 (Circulex, Nagano, Japan).
The primary end points of the present study were changes in lipid parameters including LDL cholesterol after a combination therapy of rosuvastatin (20 mg/day), ezetimibe (10 mg/day), and granulated colestimide (3.62 g/day) and safety of the therapy. The secondary end points were (1) rate to achieve LDL cholesterol levels below 100 and 120 mg/dl as target LDL cholesterol levels for secondary prevention and primary prevention of high-risk patients of cardiovascular disease as determined by the Japan Atherosclerosis Society ; (2) changes in other lipid parameters including serum levels of triglyceride, HDL cholesterol, and apolipoproteins A-I, A-II, B, C-II, C-III, and E; (3) a comparison of lipid parameters and safety between rosuvastatin monotherapy (20 mg/day) and combination therapy using rosuvastatin (10 mg/day) and ezetimibe (10 mg/day).
Values are expressed as mean ± SD unless otherwise stated. Effects of drug therapy on each variable were compared by paired t test except for triglycerides and lipoprotein(a). Effects of drug therapy and percent changes of serum levels of triglyceride and lipoprotein(a) were compared by Wilcoxon matched-pair test. All statistical analyses were performed with Prism 4.0a (GraphPad Software, Inc., San Diego, California). A p value <0.05 was considered statistically significant.
Results
Seventeen Japanese subjects with heterozygous FH were enrolled in the present study. Baseline characteristics and concomitant drug therapies are listed in Table 1 . Five of 6 diabetic patients (28%) were under hypoglycemic medical therapy and glycohemoglobin concentrations were <7.0%, which varied by only ±0.5% during the study period. No patients were treated with insulin injection therapy. Dosages of coadministered medications were kept constant during the entire study period. A 72-year-old man (patient number 15 in Table 1 ) in group 2 dropped out during phase 2 because of myalgia without an increase of serum creatinine phosphokinase, which disappeared soon after discontinuing rosuvastatin and ezetimibe.
| Patient Number | Group | Background | Risk Factors | Vascular Complications | Hypolipidemic Medication at Baseline | Concomitant Drugs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | BMI | HT | DM | Smoking | CAD | OMI | PAD | Atorvastatin (mg) | Rosuvastatin (mg) | Ezetimibe | Colestimide | Aspirin | CCB | ARB | Diuretics | ||
| 1 | 1 | 47/F | 20.1 | − | − | − | − | − | − | 40 | − | + | − | − | − | − | − |
| 2 | 1 | 49/M | 23.7 | + | − | − | + | − | − | − | 20 | + | + | + | + | + | − |
| 3 | 1 | 52/M | 24.1 | − | − | − | − | − | − | − | 20 | + | + | − | + | − | − |
| 4 | 1 | 58/M | 24.2 | − | − | − | + | + | − | − | 20 | + | + | + | − | + | − |
| 5 | 1 | 60/F | 25.7 | + | + | − | − | − | − | − | 20 | + | + | + | − | + | − |
| 6 | 1 | 63/M | 25.0 | − | + | − | + | + | − | − | 20 | + | + | + | + | − | − |
| 7 | 1 | 63/F | 20.6 | − | − | − | − | − | − | − | 15 | − | + | − | − | − | − |
| 8 | 1 | 66/M | 22.9 | + | + | − | + | + | − | − | 20 | + | + | + | + | + | + |
| 9 | 1 | 82/F | 22.0 | − | − | − | − | − | − | − | 20 | + | + | + | − | − | − |
| 10 | 2 | 60/M | 23.9 | + | + | − | + | + | + | 40 | − | + | + | + | + | − | − |
| 11 | 2 | 61/M | 24.6 | − | − | − | + | − | − | − | 10 | + | + | + | − | + | − |
| 12 | 2 | 63/M | 25.6 | + | − | + | − | − | + | 40 | − | + | + | − | − | − | − |
| 13 | 2 | 65/M | 22.1 | − | − | − | + | − | − | − | 10 | + | + | − | − | + | − |
| 14 | 2 | 73/M | 22.8 | + | − | − | + | − | − | − | 10 | + | + | + | + | + | − |
| 15 | 2 | 73/M | 23.6 | − | − | − | + | − | + | 20 | − | + | + | + | + | − | − |
| 16 | 2 | 75/M | 27.8 | + | + | − | + | + | − | − | 20 | + | + | + | + | + | + |
| 17 | 2 | 76/F | 24.7 | + | + | − | − | − | − | − | 20 | + | + | − | + | + | + |
| Mean or n | 63.9 | 23.7 | 8 | 6 | 1 | 10 | 5 | 3 | 4 | 13 | 16 | 16 | 11 | 9 | 9 | 3 | |
| SD or % | 7.4 | 1.4 | 47% | 35% | 6% | 59% | 29% | 18% | 24% | 76% | 94% | 94% | 65% | 53% | 53% | 18% | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


