Patients with mixed hyperlipidemia and at high risk of coronary heart disease may not achieve recommended low-density lipoprotein (LDL) and non–high-density lipoprotein (non-HDL) cholesterol goals on statin monotherapy. This study was designed to evaluate the efficacy and safety of a fenofibrate 160 mg/pravastatin 40 mg fixed-dose combination therapy in high-risk patients not at their LDL cholesterol goal on pravastatin 40 mg. In this 12-week, multicenter, randomized, double-blind, double-dummy, parallel-group study, after a run-in on pravastatin 40 mg, 248 patients were randomly assigned to fenofibrate/pravastatin combination therapy or to pravastatin monotherapy. Combination therapy produced significantly greater complementary decreases in non-HDL cholesterol (primary end point) than pravastatin monotherapy (−14.1% vs −6.1%, p = 0.002). Significantly greater improvements were also observed in LDL cholesterol (−11.7% vs −5.9%, p = 0.019), HDL cholesterol (+6.5% vs +2.3%, p = 0.009), triglycerides (−22.6% vs −2.0%, p = 0.006), and apolipoprotein B (−12.6% vs −3.8%, p <0.0001). Significantly more patients receiving the fenofibrate/pravastatin combination therapy than pravastatin alone achieved the LDL cholesterol (<100 mg/dl) and non-HDL cholesterol (<130 mg/dl) goals (p <0.01). Combination therapy was generally well tolerated with incidences of clinical and laboratory adverse experiences similar between the 2 groups. In conclusion, the fenofibrate 160 mg/pravastatin 40 mg fixed-dose combination therapy significantly improved the global atherogenic lipid profile in high-risk patients with mixed hyperlipidemia not controlled by pravastatin 40 mg monotherapy.
The objective of this study was to determine whether the efficacy of a fixed-dose combination of fenofibrate 160 mg/day plus pravastatin 40 mg/day was superior to that of pravastatin 40 mg/day monotherapy in decreasing non–high-density lipoprotein (non-HDL) cholesterol, a secondary target in patients with increased triglycerides, in high-risk patients with mixed dyslipidemia not at their low-density lipoprotein (LDL) cholesterol goal on pravastatin monotherapy.
Methods
This multicenter, randomized, double-blind, double-dummy, parallel-group study was conducted according to Good Clinical Practice guidelines in 41 clinical sites in 3 European countries, namely France (26 sites), Poland (10 sites), and Belgium (5 sites). The study protocol was reviewed and approved by the appropriate ethics committees and institutional review boards and all patients provided written informed consent before any study procedures were administered. Patients had to follow the National Cholesterol Education Program Adult Treatment Panel III Therapeutic Lifestyle Changes diet for ≥3 months before screening and be willing to maintain this diet for the duration of the study. Patients were required to discontinue lipid-lowering therapy before entering a 8-week run-in period during which all patients received 1 tablet of pravastatin 40 mg. Eligible patients then were randomly assigned (1:1) into 2 groups and took in double-blind, double-dummy manner 2 capsules, i.e., 1 capsule of fenofibrate 160 mg/pravastatin 40 mg and 1 capsule of placebo or 1 capsule of pravastatin 40 mg alone and 1 capsule of placebo. All treatments were administered orally with the evening meal for 12 weeks. Patients completing the 12-week efficacy study were offered enrollment into a 52-week open-label extension study, which will be the subject of a future publication.
This study enrolled men and women ≥18 years of age with documented combined hyperlipidemia and high cardiovascular risk according to Adult Treatment Panel III definitions. Patients were required to meet ≥1 of the following criteria: (1) history of coronary heart disease, (2) history of other clinical forms of atherosclerotic disease, (3) diabetes, or (4) coronary heart disease risk >20% over 10 years as determined by the Framingham risk calculation. After the active treatment run-in period with pravastatin 40 mg, subjects with LDL cholesterol levels ≥100 mg/dl and fasting triglyceride concentrations ≥150 and ≤400 mg/dl at baseline (week 0) were randomized to fenofibrate 160 mg/pravastatin 40 mg or pravastatin 40 mg for 12 weeks. Main exclusion criteria were history of any sensitivity or allergy to statins and/or fibric acid derivatives; history of an acute cardiovascular event within 6 months before enrollment; uncontrolled hypertension (systolic >160 mm Hg or diastolic >95 mm Hg); history of malignancy; uncontrolled hypothyroidism; current active liver disease (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] >2 times the upper limit of normal [ULN]); creatine kinase >3 times ULN; creatinine clearance <50 ml/min or any significant renal disease; type 1 diabetes or type 2 diabetes with poor control (hemoglobin A1c level >8.5%) or requiring insulin; use of prohibited concomitant medications (including oral estroprogestin contraceptives); and compliance <80% during the run-in period. Women who were pregnant or breastfeeding or of childbearing potential but not using contraception were also excluded from the study.
The primary efficacy end point was mean percent change in non-HDL cholesterol from baseline (i.e., after 8 weeks under pravastatin 40 mg) to the end of the efficacy period. Baseline value was defined as the average of measurements obtained 1 week before randomization and the day of randomization. The study end point value was defined as the last measurement after baseline measurement during the 12-week double-blind period. Secondary efficacy end points included percent changes from baseline in LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides, LDL size, apolipoprotein B, apolipoprotein A-I, apolipoprotein B/apolipoprotein A-I ratio, high-sensitivity C-reactive protein, and fibrinogen and percentage of patients achieving the Adult Treatment Panel III LDL cholesterol and non-HDL cholesterol goals after 12 weeks of treatment. Safety was assessed by monitoring clinical adverse events and vital signs in all randomized patients and laboratory adverse events in all treated patients with ≥1 on-treatment measurement. Prespecified safety parameters included ALT and/or AST increases >3 times ULN, creatine kinase increases ≥5 and <10 times ULN, creatine kinase increases ≥10 times ULN without or with muscular symptoms (myopathy), and creatinine >20 mg/L (177 μmol/L) with clearance of creatinine <50 ml/min.
Fasting blood samples were collected at weeks −8 (beginning of run-in period), −1, 0 (randomization), 4, 8, and 12 for lipid profile analysis and for clinical chemistry (including ALT, AST, creatine kinase, and serum creatinine). LDL cholesterol levels were determined using the Friedewald formula, with the exception of visits in which triglyceride levels were >400 mg/dl, when a beta-quantification measurement of LDL cholesterol was used. LDL peak particle size was assessed with segmented gradient gel electrophoresis. High-sensitivity C-reactive protein and fibrinogen were quantified by immunonephelometry. All clinical laboratory analyses were performed at a central laboratory (Eurofins Medinet SAS, Plaisir, France). Safety was assessed by frequency of adverse events and abnormal laboratory data. Each investigator was required to make a causality assessment of the relation of the adverse event to the study drugs and whether it constituted a serious adverse event. All study personnel, including investigators, study-site personnel, patients, monitors, and central laboratory personnel remained blinded to treatment allocation throughout the study. Study personnel remained blinded until the study was completed and the data file was locked. Compliance was assessed at each visit by counting the number of returned tablets; patients were considered noncompliant if they used <80% of the prescribed number of tablets.
For the sample size calculation, because reference data on non-HDL cholesterol levels were not available, hypotheses were based on LDL cholesterol levels for effect of the fenofibrate/pravastatin combination. It was expected that with 140 patients (70 per group), there would be 90% power to detect a between-treatment group difference of 6 percentage points in the mean percentage change in LDL cholesterol from baseline to week 12 (1-tailed test on alpha = 0.05), considering an SD of 20 mg/dl. Statistical power from the calculation based on LDL cholesterol levels was a fortiori sufficient considering the non-HDL cholesterol levels. For the non-HDL cholesterol primary efficacy end point, a 2-sided test on significance levels of 5% was performed to demonstrate the superiority of the fenofibrate/pravastatin combination versus pravastatin. Assuming a drop-out rate of about 40% of patients before the end of the safety period (64 weeks), 240 patients were required to be randomly assigned. Efficacy was evaluated by randomized treatment in the intention-to-treat population, which consisted of all patients with a baseline lipid evaluation and ≥1 lipid measurement after baseline and who took ≥1 dose of active drug during the double-blind period. Analyses used the last-available-observation-carried-forward approach for patients with missing data. Mean percent changes from baseline in lipid and lipoprotein levels were compared between treatment groups using an analysis of covariance, including the baseline value as a covariable. If the distribution of the data was nongaussian, a nonparametric test was used. For the proportion of patients achieving LDL cholesterol and/or non-HDL cholesterol goals, comparisons between treatment groups were assessed using chi-square test. Occurrence of adverse events during the 12-week double-blind period was compared between treatment groups using chi-square test (or Fischer’s exact test, if necessary). Duration of exposure (in days) and compliance (in percentages) were compared between the 2 treatment groups using Student’s t test (or Wilcoxon-Mann-Whitney test, if necessary).
Results
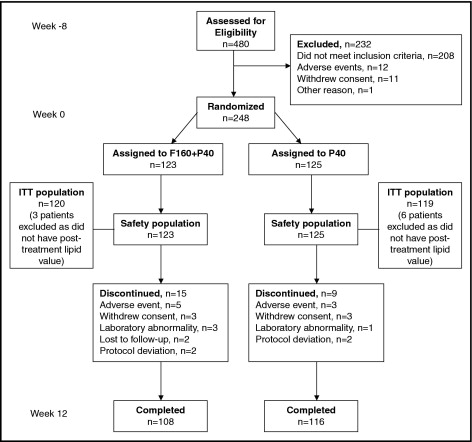
Flow of participants through the study is presented in Figure 1 . Of 480 patients who entered the run-in period on pravastatin 40 mg, 248 were randomly assigned (123 to fenofibrate 160 mg plus pravastatin 40 mg combination therapy and 125 to pravastatin 40 mg monotherapy). There were no clinically meaningful differences in distribution of baseline characteristics between treatment groups, including demographics, risk factors, and lipid values ( Tables 1 and 2 ). Baseline characteristics showed that 73% of patients were <65 years of age and 97% of women had no childbearing potential. Of the 248 randomized patients, only 1 patient in the pravastatin group had a 10-year risk ≤20% for coronary heart disease. Of the 239 patients from the intention-to-treat population, 99.6% received ≥1 concomitant treatment from the selection visit to week 12. Further, 69.5% of patients were receiving statins before the study and stopped taking it when entering the run-in pravastatin 40 mg phase. At the end of the 12-week study, 95% and 97% of patients were compliant with fenofibrate/pravastatin and pravastatin treatments, respectively, and mean duration of exposure to treatment was 86 days.
| Parameter | Fenofibrate 160 mg + Pravastatin 40 mg | Pravastatin 40 mg | Total |
|---|---|---|---|
| (n = 123) | (n = 125) | (n = 248) | |
| Age (years) | 57.8 ± 9.3 | 58.1 ± 9.3 | 58.0 ± 9.3 |
| Men | 69.9% | 70.4% | 70.2% |
| Body mass index (kg/m 2 ) | 29.3 ± 4.1 | 29.9 ± 4.3 | 29.6 ± 4.2 |
| Waist circumference (cm) | 100.3 ± 10.8 | 102.3 ± 11.0 | 101.3 ± 10.9 |
| Clinical atherosclerosis | |||
| Known coronary heart disease | 75 (61%) | 72 (58%) | 147 (59%) |
| Other ⁎ | 18 (15%) | 30 (24%) | 48 (19%) |
| Diabetes mellitus | 32 (26%) | 36 (29%) | 68 (27%) |
| Current smoker | 34 (28%) | 28 (22%) | 62 (25%) |
| Hypertension (≥140/90 mm Hg or on antihypertensive medication) | 93 (76%) | 97 (78%) | 190 (77%) |
| Duration of hyperlipidemia (years) | 7.2 ± 6.4 | 7.2 ± 5.4 | 7.2 ± 5.9 |
⁎ Peripheral arterial disease, abdominal aortic aneurysm, and/or symptomatic carotid artery disease.
| Parameter | Fenofibrate 160 mg + Pravastatin 40 mg (n = 120) | Pravastatin 40 mg (n = 119) | p Value ⁎ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Mean Percent Change | p Value | Baseline | Week 12 | Mean Percent Change | p Value | ||
| Primary end point | |||||||||
| Non–high-density lipoprotein cholesterol (mg/dl) | 183 ± 30 | 156 ± 45 | −14.1 | <0.0001 | 187 ± 31 | 174 ± 37 | −6.1 | <0.001 | 0.002 |
| Secondary end points | |||||||||
| Low-density lipoprotein cholesterol (mg/dl) | 138 ± 27 | 121 ± 32 | −11.7 | <0.0001 | 141 ± 29 | 131 ± 34 | −5.9 | 0.001 | 0.019 |
| High-density lipoprotein cholesterol (mg/dl) | 48 ± 8 | 51 ± 10 | + 6.5 | <0.0001 | 47 ± 8 | 48 ± 9 | +2.3 | 0.045 | 0.009 |
| Total cholesterol (mg/dl) | 231 ± 32 | 207 ± 45 | −9.9 | <0.0001 | 234 ± 32 | 223 ± 38 | −4.4 | 0.001 | 0.006 |
| Triglycerides (mg/dl) | 235 ± 76 | 173 ± 117 | −22.6 | <0.0001 | 239 ± 80 | 225 ± 87 | −2.0 | 0.64 | 0.001 |
| Apolipoprotein A-I (mg/dl) | 138 ± 18 | 145 ± 23 | +5.5 | <0.0001 | 138 ± 17 | 141 ± 19 | +2.8 | 0.004 | 0.058 |
| Apolipoprotein B (mg/dl) | 111 ± 19 | 96 ± 23 | −12.6 | <0.0001 | 113 ± 21 | 108 ± 25 | −3.8 | 0.012 | <0.0001 |
| Apolipoprotein B/apolipoprotein A-I | 0.82 ± 0.17 | 0.68 ± 0.20 | −16.2 | <0.0001 | 0.83 ± 0.19 | 0.78 ± 0.22 | −6.1 | <0.001 | <0.0001 |
| Fibrinogen (g/L) | 3.48 ± 0.60 | 3.12 ± 0.54 | −8.8 | <0.0001 | 3.39 ± 0.67 | 3.42 ± 0.73 | +1.4 | 0.42 | <0.0001 |
| High-sensitivity C-reactive protein (mg/L) † | 1.82 | 1.40 | −23.2 | 0.005 | 1.73 | 1.84 | +1.3 | 0.053 | 0.001 |
⁎ For percent change from baseline with fenofibrate 160 mg/pravastatin 40 mg group versus pravastatin 40 mg group at week 12.
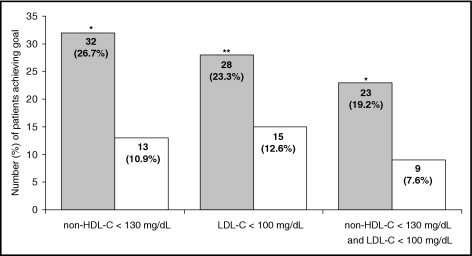
Coadministration of fenofibrate 160 mg with pravastatin 40 mg produced a significantly greater decrease in non-HDL cholesterol after 12 weeks of treatment compared to pravastatin 40 mg, with a between-treatment difference of −8% (p = 0.002; Table 2 ). The 2 study treatments had a maximum effect on non-HDL cholesterol levels after 4 weeks. Combination drug therapy also significantly positively affected plasma concentrations of LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides, and apolipoprotein B compared to pravastatin monotherapy at week 12 ( Table 2 ). Apolipoprotein A-I increased by 5.5% and 2.8% in the combination therapy and monotherapy groups, respectively (p = 0.058; Table 2 ). A significant decrease in apolipoprotein B/apolipoprotein A-I ratio was observed in the combination therapy group compared to the monotherapy group (p <0.0001; Table 2 ). LDL size was evaluated in 103 and 108 patients from the combination therapy and monotherapy groups, respectively. LDL peak particle size increased significantly in the combination therapy group (+1.54 ± 1.75, mean percent change ± SEM, p <0.0001), with no significant change in the monotherapy group (−0.16 ± 1.81, p = 0.15) and a highly significant between-treatment difference (p <0.0001). After 12 weeks of treatment, fibrinogen significantly decreased in the combination therapy group, with a significant between-treatment difference (p <0.0001; Table 2 ). A median percent decrease in high-sensitivity C-reactive protein was observed only in the combination therapy group ( Table 2 ). Significantly more patients achieved the Adult Treatment Panel III LDL cholesterol goal (<100 mg/dl) at week 12 with combination therapy versus monotherapy (p <0.05; Figure 2 ). In addition, at week 12, significantly more patients achieved the non-HDL cholesterol goal (<130 mg/dl) in the combination therapy than in the monotherapy group (p <0.01; Figure 2 ). The 2 Adult Treatment Panel III goals (non-HDL cholesterol <130 mg/dl and LDL cholesterol <100 mg/dl) were achieved by a significantly higher percentage of patients in the combination therapy than in the monotherapy group (p <0.01; Figure 2 ).