A first pilot study of interleukin-1 blockade in ST-segment elevation acute myocardial infarction showed improved remodeling. In the present second pilot study, we enrolled 30 patients with clinically stable ST-segment elevation acute myocardial infarction randomized to anakinra, recombinant interleukin-1 receptor antagonist, 100 mg/day for 14 days or placebo in a double-blind fashion. The primary end point was the difference in the interval change in left ventricular (LV) end-systolic volume index between the 2 groups within 10 to 14 weeks. The secondary end points included changes in the LV end-diastolic volume index, LV ejection fraction, and C-reactive protein levels. No significant changes in end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction were seen in the placebo group. Compared to placebo, treatment with anakinra led to no measurable differences in these parameters. Anakinra significantly blunted the increase in C-reactive protein between admission and 72 hours (+0.8 mg/dl, interquartile range −6.4 to +4.2, vs +21.1 mg/dl, interquartile range +8.7 to +36.6, p = 0.002), which correlated with the changes in LV end-diastolic volume index and LV end-systolic volume index at 10 to 14 weeks (R = +0.83, p = 0.002, and R = +0.55, p = 0.077, respectively). One patient in the placebo group (7%) died. One patient (7%) in the anakinra group developed recurrent acute myocardial infarction. More patients were diagnosed with new-onset heart failure in the placebo group (4, 27%) than in the anakinra group (1, 7%; p = 0.13). When the data were pooled with those from the first Virginia Commonwealth University-Anakinra Remodeling Trial (n = 40), this difference reached statistical significance (30% vs 5%, p = 0.035). In conclusion, interleukin-1 blockade with anakinra blunted the acute inflammatory response associated with ST-segment elevation acute myocardial infarction. Although it failed to show a statistically significant effect on LV end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction in this cohort of clinically stable patients with near-normal LV dimensions and function, anakinra led to a numerically lower incidence of heart failure.
Acute myocardial infarction with ST-segment elevation (STEMI) is characterized by the loss of a significant amount of ischemic myocardium due to atherothrombosis of a major coronary artery. Myocardial ischemia and necrosis trigger a sterile inflammatory response, responsible for the amplification of the injury and promotion of adverse cardiac remodeling and heart failure. Strategies aimed at inhibiting interleukin (IL)-1, 1 of the principle mediators in the inflammatory response, have been shown to limit left ventricular (LV) enlargement and dysfunction in animal models of large anterior wall infarction. More recently, we have reported data on the safety and efficacy of anakinra, a recombinant human IL-1 receptor antagonist, in 10 patients with STEMI. We now present the results of a second randomized, double-blind, pilot trial comparing anakinra and placebo in terms of LV enlargement and dysfunction in 30 clinically stable patients with STEMI.
Methods
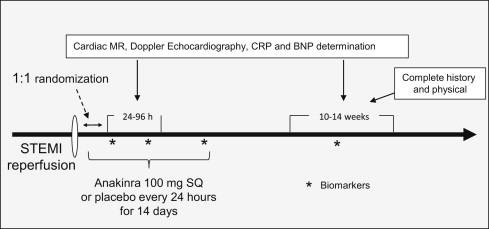
The study design was registered in www.clinicaltrials.gov ( NCT01175018 ). An exemption for investigational new drug use was granted by the Food and Drug Administration according to the current regulations [CFR 312.2(b)]. The Virginia Commonwealth University institutional review board approved the study, and all patients provided written informed consent. Starting September 1, 2010, consecutive patients presenting to our institution with suspected STEMI were screened for enrollment ( Figure 1 ). The inclusion criteria were age >18 years, acute (<24 hours) onset of chest pain, new or presumably new ST-segment elevation (>1 mm) in ≥2 anatomically contiguous leads, and planned or completed angiography for urgent percutaneous coronary intervention. The exclusion criteria were a lack of informed consent; cardiac arrest; unsuccessful percutaneous revascularization or the need for urgent surgical revascularization; hemodynamic instability (requiring the use of an intra-aortic balloon pump, vasopressors, or inotropes); previous Q-wave infarction or pre-existing congestive heart failure stage C/D, New York Heart Association class IV; severe LV dysfunction (LV ejection fraction <20%); or severe aortic or mitral valve disease; contraindications to magnetic resonance imaging; pregnancy; chronic infections, autoinflammatory or autoimmune disease, or cancer; and recent (<14 days) use of anti-inflammatory drugs (nonsteroidal anti-inflammatory drugs excluded). Cardiac magnetic resonance (CMR) and Doppler echocardiographic studies were performed 24 to 96 hours after admission and 10 to 14 weeks later. High-sensitivity C-reactive protein (CRP) and brain-type natriuretic peptide were measured at admission, 72 hours, 14 days, and 10 to 14 weeks. Serial cardiac markers were obtained as clinically indicated. Clinical follow-up was performed during hospitalization and at weeks 2 and 12. The investigational pharmacist performed randomization using a dedicated randomization algorithm obtained from randomization.com (seed no. 7408, created July 23, 2010). For each patient, the pharmacist prepared a set of 14 syringes with the content of 100 mg of anakinra (Kineret, Swedish Orphan Biovitrum, Stockholm, Sweden) in 0.67 ml or matching NaCl 0.9% placebo. The syringes were indistinguishable. Treatment consisted of 14 daily subcutaneous injections. A complete blood cell count was obtained at admission, daily for 3 days, and again at 14 days. The CMR studies were obtained in the VCU cardiac magnetic resonance imaging suite using a Siemens Avanto 1.5 Tesla magnet, as previously described. In brief, contiguous, 6-mm, steady-state, free precession cine images with 2-mm gaps were obtained from the mitral valve ring through the cardiac apex. The LV end-systolic volume, LV end-diastolic volume, and LV ejection fraction were computed by tracing the LV contours using this stack of 10 to 12 short-axis slices and aided by the software CMR42 (Circle Cardiovascular Imaging, Calgary, Alberta, Canada). To visualize the areas of infarction and no reflow, late gadolinium-enhanced imaging was performed, beginning 10 minutes after contrast administration. The infarct size was calculated using the CMR42 software and the full-width-at-half-maximum technique. The dimensions were indexed to the body surface area. All measurements and calculations were performed at the end of the study jointly by 2 investigators, who were unaware of the patient treatment and decisions were made in consensus. The white blood cell count, brain-type natriuretic peptide, troponin I, creatine kinase-MB, creatinine, and other routine tests were performed in the Virginia Commonwealth University Health System Pathology Laboratories. The determination of high-sensitivity CRP levels was performed by LabCorp using high-sensitivity rate nephelometry. The primary end point was the difference in interval changes in the LV end-systolic volume index from baseline to follow-up comparing the anakinra- and placebo-treated patients. The secondary end points included the difference in interval changes in the LV end-diastolic volume index, LV ejection fraction, and infarct size. The clinical events were adjudicated by 3 investigators unaware of treatment allocation and based on the documentation available in the chart, and consensus was needed for all determinations. For the purpose of the present analysis, data on clinical events from the first pilot study were retrieved and combined with the data from the present second study. From the results of the Virginia Commonwealth University-Anakinra Remodeling Trial (VCU-ART) study, showing a median difference of 5 ml/m 2 in the interval changes in LV end-systolic volume index favoring anakinra, with a SD of 6 ml/m 2 , we calculated a sample size of 30 patients to provide a power of >80% (α 0.05), allowing for a 20% loss to follow-up. The values are reported as the median and interquartile range for potential deviation from the gaussian distribution. The differences between the 2 groups were computed using the Wilcoxon test for continuous variables and Fisher’s exact test for discrete variables. The Spearman correlation test was used to evaluate the correlation between the 2 variables. The differences in interval changes between groups were compared using a random-effect general linear model for repeated measures analyzing the effects of time and group allocation. Kaplan-Meier curves for survival and event-free survival were constructed and compared using the log-rank Mantel-Cox test. Unadjusted p values are reported, with statistical significance set at the 2-tailed 0.05 level. The analyses were completed using SPSS, version 19.0 (IBM, Armonk, New York).
Results
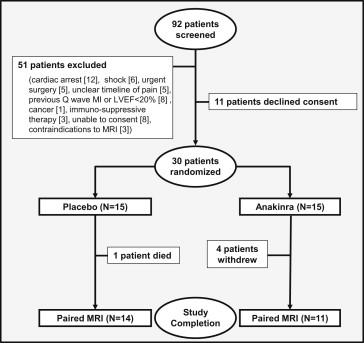
From September 2010 to May 2012, we screened 92 patients admitted with STEMI and enrolled 30 patients ( Figure 2 ). No significant differences were found in age, gender, ethnicity, risk factors, or clinical characteristics between the 2 groups ( Table 1 ). Three patients in the anakinra group requested discontinuation of treatment during the first 2-week study (20%) and none in the placebo group (0%, p = 0.073). One patient in the anakinra group completed follow-up but refused to undergo a repeat CMR study, and one in the placebo group died. A total of 25 patients (11 in the anakinra group and 14 in the placebo group) completed the study and had paired CMR studies. The interval from admission to the first or second CMR study was not statistically different between the 2 groups (46 hours, range 31 to 68, vs 44 hours, range 38 to 52, for the anakinra and placebo groups, respectively, p = 0.33 for the initial CMR study; and 91 days, range 84 to 98, vs 88 days, range 82 to 92, p = 0.12 for the follow-up CMR). The infarct size was 21.2% in the anakinra group and 18.0% in the placebo group (p = 0.81), and evidence of no-reflow was virtually absent in all patients ( Table 1 ). One patient in the placebo group had pathologic wall thinning and cardiac rupture (see the “Clinical Events” section). Another patient, also in the placebo group, had a large apical thrombus requiring anticoagulation. No signs were seen of impaired healing, pseudoaneurysm, thrombus, pathologic infarct wall thinning, or signs of impending rupture in any of the anakinra-treated patients. No significant differences were seen in the use of guideline-recommended treatments at discharge (data not shown). All but 2 patients received target coronary vessel stenting, with 1 patient in the anakinra group receiving balloon angioplasty without stenting and 1 patient, also in the anakinra group, who had nonocclusive coronary artery disease after successful fibrinolysis. Six patients, four in the anakinra and two in the placebo group, underwent staged angioplasty for nonculprit lesion during the index hospitalization. At 10 to 14 weeks of follow-up, no significant changes were seen in LV end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction compared to baseline in the placebo group or the anakinra group ( Table 1 and Table E1 ). No significant differences were seen in the interval changes in LV end-systolic volume index (primary end point), LV end-diastolic volume index, LV ejection fraction, or LV mass between the placebo- and anakinra-treated patients ( Table 1 , Table E1 , and Figure 3 ). The CRP levels at admission were not statistically different in the patients who were randomized to anakinra (7.0 mg/dl, range 2.3 to 8.7, vs placebo 4.3 mg/dl, range 2.2 to 7.5, p = 0.30). The interval change in CRP levels between admission and 72 hours, a measurement of the acute inflammatory response, was significantly blunted in the anakinra group (p = 0.002 vs placebo, respectively, Figure 4 ). In patients treated with anakinra, the interval change in CRP levels within 72 hours correlated with the change in LV end-diastolic volume index (R = +0.83, p = 0.002) and LV end-systolic volume index (R = +0.55, p = 0.077) on CMR studies at 10 to 14 weeks. The CRP levels had returned to admission levels by day 14 in the placebo-treated patients, and treatment with anakinra failed to reduce the 14-day CRP levels beyond the reduction seen in the placebo group ( Figure 4 ). The changes in LV end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction were not correlated with the interval to presentation, angioplasty, or drug treatment in either group nor were they affected by infarct location, culprit vessel, or extent of coronary artery disease (data not shown). No difference in the changes in brain-type natriuretic peptide levels between groups was observed (data not shown). Clinical data were available for all patients throughout the study period. Adverse clinical events are reported in Table 1 and summarized in Table E2 . The median Thrombolysis In Myocardial Infarction risk score was 3 in both groups, with a predicted 30-day mortality of 4.4%. One patient in the placebo group (7%) died 38 days after admission from complications related to cardiac rupture and cardiogenic shock requiring emergent cardiac surgery. One patient in the anakinra group (7%) experienced a recurrent non–STEMI due to subocclusive stent thrombosis 4 days after initial stent implantation. Two patients in each group had adverse events requiring treatment discontinuation. Four patients in the anakinra group (27%) and two in the placebo group (13%, p = 0.39) had evidence of an infection during treatment; of these cases, infection was considered serious (requiring intravenous antibiotic administration, new hospitalization, or prolongation of the hospitalization) in 2 patients (13%) in each group. In the 4 patients treated with anakinra who experienced infection, 2 with serious infection (urosepsis) had active treatment suspended between day 5 and 7, and both had a full recovery from infection. The other 2 had upper respiratory infections. Of the 2 patients treated with placebo, 1 with urosepsis had full recovery, and 1 developed nosocomial pneumonia and died while recovering from cardiac surgery and sepsis. Injection site reactions were infrequent in both groups, with 1 patient in the anakinra group with mild injection site reactions withdrawing from treatment. More patients were diagnosed with new-onset heart failure (American Heart Association/American College of Cardiology stage C, New York Heart Association class III-IV), defined as new or worsening shortness of breath and a new or an increased requirement for diuretics, in the placebo group (4, 27%) than in the anakinra group (1, 7%; p = 0.13). When the data were pooled with the data from the first VCU-ART study (n = 40), this difference reached statistical significance (30% vs 5%, respectively, p = 0.035; Figure 5 ). One patient in the placebo group (7%) required hospital admission for decompensated heart failure.
| Age (yrs) | Gender | Ethnicity | Hypertensions | Diabetes | Interval from Chest Pain to PCI (min) | Interval from Chest Pain to Drug (min) | CAD (Culprit) | Initial TIMI Flow | Final TIMI Flow | Coronary Stenting | Infarct Size (%) | Adverse Clinical Events | Change in LVESVi (ml/m 2 ) | Change in LVEF (abs%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | |||||||||||||||
| 1 | 35 | F | Black | Yes | No | 155 | 612 | RCA | 0 | 3 | Yes | 9.2 | Bleeding requiring medical attention | −10 | +6 |
| 2 | 39 | M | White | Yes | Yes | 90 | 398 | LAD | 1 | 3 | Yes | 23.6 | Injection site pain, bleeding requiring medical attention | +8 | −3 |
| 3 | 50 | M | Black | Yes | No | 60 | 475 | RCA | 0 | 3 | Yes | 18.0 | Infection (serious) | +3 | −9 |
| 4 | 51 | M | White | Yes | No | 330 | 480 | LAD | 0 | 2 | Yes | 32.9 | Heart failure | +8 | +8 |
| 5 | 52 | M | White | Yes | Yes | 30 | 250 | LAD | 0 | 3 | Yes | 9.3 | Heart failure | −5 | 0 |
| 6 | 57 | M | Black | Yes | No | 408 | 605 | RCA | 0 | 3 | Yes | 10.9 | Other (presyncope) | +12 | −5 |
| 7 | 57 | M | Black | Yes | No | 168 | 478 | LCX | 0 | 3 | Yes | 18.0 | Other (eosinophilia) | 0 | 0 |
| 8 | 59 | M | Black | No | No | 48 | 450 | LAD | 0 | 3 | Yes | 34.5 | Heart failure | −10 | +5 |
| 9 | 60 | M | White | No | No | 630 | 700 | LAD | 0 | 3 | Yes | 30.7 | Other (LV thrombus) | +10 | −7 |
| 10 | 63 | M | Black | No | No | 125 | 325 | RCA | 0 | 3 | Yes | 37.0 | Bleeding requiring medical attention | −2 | −4 |
| 11 | 65 | F | White | No | No | 367 | 585 | RCA | 0 | 3 | Yes | 8.0 | None | −10 | +8 |
| 12 | 65 | M | White | Yes | Yes | 60 | 300 | RCA | 0 | 3 | Yes | 15.5 | Injection site pain | +2 | −5 |
| 13 | 66 | M | Black | Yes | No | 600 | 775 | OM2 | 0 | 3 | Yes | 11.5 | Other (hyperkalemia) | −3 | +5 |
| 14 | 71 | M | White | Yes | No | 180 | 360 | RCA | 0 | 3 | Yes | 23.9 | Bleeding requiring medical attention, urinary retention | +7 | −3 |
| 15 | 83 | M | White | Yes | No | 540 | 720 | LCX | 0 | 2 | Yes | NA | Cardiac rupture, heart failure, repeat PCI, sepsis, death | NA | NA |
| Anakinra | |||||||||||||||
| 1 | 46 | M | White | No | No | 165 | 1,215 | LAD | 0 | 3 | Yes | NA | None | NA | NA |
| 2 | 46 | F | Black | Yes | No | 55 | 865 | RCA | 3 | NA | No | 21.4 | Infection (minor) | +4 | −1 |
| 3 | 48 | F | Black | Yes | Yes | 180 | 630 | OM1 | 0 | 3 | No | 23.4 | Infection (serious) | +4 | +12 |
| 4 | 50 | M | White | No | No | 120 | 375 | RCA | 0 | 3 | Yes | 20.0 | Recurrent MI, repeat PCI | −1 | +2 |
| 5 | 50 | F | White | Yes | Yes | 150 | 600 | OM2 | 0 | 3 | Yes | 23.2 | Injection site pain | −4 | +5 |
| 6 | 56 | 1F | Black | No | Yes | 144 | 258 | RCA | 0 | 3 | Yes | 32.1 | Infection (minor) | +6 | −11 |
| 7 | 57 | F | Black | Yes | No | 724 | 1,380 | RCA | 0 | 3 | Yes | 14.8 | Other (thrombocytopenia) | −15 | +30 |
| 8 | 57 | M | White | Yes | No | 75 | 210 | LAD | 0 | 3 | Yes | 18.0 | Injection site pain | +4 | +2 |
| 9 | 57 | M | Black | No | No | 120 | 315 | RCA | 2 | 3 | Yes | 21.2 | None | +1 | −5 |
| 10 | 60 | M | White | No | No | 150 | 360 | RCA | 1 | 3 | Yes | 22.9 | Injection site pain | −8 | +10 |
| 11 | 60 | M | Black | No | No | 606 | 788 | LAD | 0 | 3 | Yes | NA | None | NA | NA |
| 12 | 66 | M | White | Yes | No | 35 | 491 | RCA | 0 | 3 | Yes | NA | None | NA | NA |
| 13 | 68 | M | White | Yes | No | 940 | 1,134 | OM3 | 0 | 3 | Yes | 15.7 | None | +13 | −2 |
| 14 | 80 | F | White | No | No | 900 | 1,305 | RCA | 0 | 3 | Yes | NA | Heart failure | NA | NA |
| 15 | 86 | M | Black | No | No | 40 | 247 | PDA | 0 | 3 | Yes | 13.0 | Infection (serious), bleeding requiring medical attention | −2 | +2 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


