Ventricular arrhythmias (VAs) in patients with catecholaminergic polymorphic ventricular tachycardia type 1 (CPVT1) are triggered at an individual and reproducible heart rate (HR) during exercise. Long-term effects of exercise on arrhythmia threshold in CPVT1 are not known. To investigate whether exercise training (ET) is feasible in patients with CPVT1, 13 patients with CPVT1 and confirmed genetic mutations performed bicycle exercise testing with maximal oxygen uptake (VO 2 max) measurements at baseline and after 13 weeks. The threshold HR for VA was defined as the HR when bigeminal ventricular extrasystoles or more severe VAs occurred. Six patients were enrolled in a 12-week high-intensity ergometer bicycle ET program (ET patients) with 60 min exercise sessions 3 times per week. The remaining 7 patients with CPVT1 were included as “sedentary” control (SED) patients complying with current recommendations to restrain from high-intensity physical activity. ET patients completed 28 ± 3 exercise sessions (78 ± 8% program completion) with 13 ± 3% increase in VO 2 max versus baseline (20.2 ± 1.6 vs 17.9 ± 1.3 ml/kg/min, p <0.05). No adverse events occurred. Baseline threshold for VA was 100 ± 6 beats/min in ET patients and 135 ± 4 beats/min in SED patients. After the training period, threshold HR for VA was 111 ± 10 beats/min in ET patients and 123 ± 6 beats/min in SED patients. The threshold for VA increased in ET compared with SED patients (+11 vs −12 beats/min, p <0.05). In conclusion, patients with CPVT1 benefitted from individualized ET with improved aerobic capacity and increased threshold HR for VA compared with SED patients.
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited cardiac disorder in which patients have structurally normal hearts but increased risk of stress-induced ventricular arrhythmias (VAs) and sudden cardiac death because of mutations in the cardiac “ryanodine receptor 2” (RyR2; CPVT1) or “calsequestrin 2” (CPVT2). With increasing exercise intensity, patients with CPVT1 exhibit an increased frequency and severity of ventricular extrasystoles (VESs) that may develop into bidirectional or polymorphic ventricular tachycardias (VTs). Onset of bigeminal VES or more severe arrhythmias occur at a heart rate (HR) that is individual and reproducible for each patient. Patients with CPVT1 are advised to restrain from high-intensity physical activity and competitive sports. However, a beneficial effect from regular exercise training (ET) can be hypothesized based on results from animal studies: regular ET has been shown to reduce the propensity for VA in a mouse model of CPVT2. Furthermore, exercise counteracts RyR2 dysfunction in animal models of diabetes, probably through reduced activation of Ca 2+ -calmodulin–dependent kinase type II, and pharmacologic inhibition of this kinase reduced the propensity for VA in a mouse model of CPVT1. Based on these effects of ET, we hypothesized that patients with CPVT1 could exercise safely at an individual HR below the threshold HR for VA with improvement in aerobic capacity and with beneficial effects on the propensity for arrhythmias.
Methods
Patients diagnosed with CPVT1 were enrolled from the Department of Cardiology, Oslo University Hospital, Rikshospitalet, Oslo, Norway. All patients had confirmed CPVT1-associated RyR2 mutations and typical symptoms or VA during exercise testing. Exclusion criteria were patient characteristics preventing ET, such as disabilities, co-morbidity, and recent or pending surgery. All patients were invited to participate in a 12-week ET program (ET patients). Patients who were a priori unable to participate in the ET program because of their work situation, family obligations, or geography were included as “sedentary” control (SED) patients, with unaltered advice to avoid intense physical and emotional stress. Written informed consent was obtained from all patients. The study complied with the Declaration of Helsinki and was approved by the Regional Committee for Medical and Health Research Ethics (REC-South-East; REC ID 2011/19297).
Clinical history and examination, blood sampling, and standard echocardiography were performed in all patients before each exercise test. Maximum oxygen uptake (VO 2 max) was only measured in 4 of 7 SED patients. Holter monitoring (Medilog AR4 Plus, Schiller AG, Baar, Switzerland) was performed at baseline, 12 weeks (week 13), and 40 weeks (6 months) after initiation of the exercise program. Recordings were analyzed using Medilog Darwin professional software (Medilog, Schiller AG, Baar, Switzerland).
Aerobic exercise capacity was tested for all ET patients at baseline (week 0), twice during the exercise period (weeks 3 and 9), immediately after (week 13), and 6 months after study completion. SED patients performed exercise testing at baseline and after 12 to 36 weeks. Aerobic capacity was tested with a standard bicycle exercise test (Schiller CS-200 Ergo-Spiro from Diacor, Schiller AG, Baar, Switzerland). Baseline workload was set to 25 to 50W with 25W increase every second minute until subjective exhaustion due to dyspnea or leg discomfort, regardless of the occurrence of arrhythmias. Heart rhythm was monitored using a 12-lead electrocardiograph (Schiller SDS-200, Schiller AG, Baar, Switzerland). Blood pressure and VO 2 derived from standard O 2 /CO 2 measurements were continuously monitored. Baseline HR, maximum HR, VO 2 max, and blood pressure were recorded (Schiller LF8, Schiller AG, Baar, Switzerland).
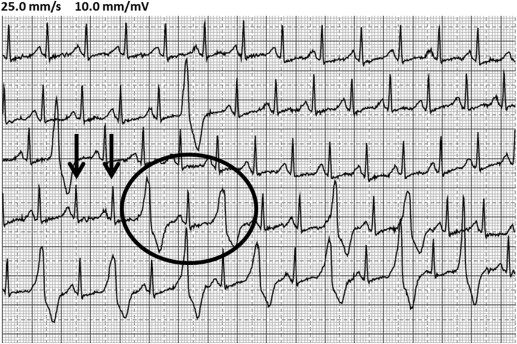
The threshold HR for VA was defined as the HR at which VES in bigeminy, couplets, triplets, nonsustained VT, or sustained VT occurred during the exercise test. Alternatively, if only single VES or no arrhythmias occurred, maximal achieved HR was considered the threshold ( Figure 1 ). VO 2 max measurements and threshold HR for arrhythmias were analyzed individually by 2 of the investigators (JS and RM) blinded to patient identity and study group.
ET patients performed a 12-week exercise program, consisting of 3 sessions of high-intensity ergometer bicycling per week. A similar training protocol with a 12-week program increased VO 2 max by 14% in patients with heart failure. Each exercise session consisted of 4 intervals of 6 minutes, interspersed with 3-minute active rest periods. Ten-minute warm-up and cool-down periods were included. In total, each session lasted 53 minutes. The target HR during intervals was individualized based on the threshold HR for VA. The threshold HR for VA in each patient was adjusted after each exercise test during the study. A safety margin of 5 beats/min was subtracted from the HR in cases when arrhythmias occurred, giving a “modified threshold HR for arrhythmias.” The target HR during intervals was calculated as 80% to 90% of the modified threshold HR, whereas the target HR for the active rest periods was 50% to 60% of the modified threshold HR. All training sessions were standardized with regard to instructors, music, facilities, and ergometer bicycles. A physician and a nurse equipped with an external defibrillator were present during all training sessions.
Clinical measures at baseline and after 12 weeks of exercise within each study group were compared using Student paired t test, whereas comparisons between the ET patients and SED patients were performed using unpaired t test. The development in threshold HR for VA was compared using 1-sided test. p <0.05 was considered significant.
Results
Thirteen patients with CPVT1 were included; 6 participated in the exercise program (ET patients) and 7 patients were included as controls (SED patients). Patients from 4 different families were included in the study, with 3 different mutations: G2337V 46 RyR2, R172Q 8 RyR2, and G4671V 97 RyR2. Family relations, mutations, and clinical characteristics are given in Table 1 and Table 2 . Clinical characteristics did not differ between the 2 groups and did not change during the study period, except for an increase in body mass index (p = 0.04) in SED patients.
| Patient ET | Age (yrs) | Mutation | Medication (Dose) | Body Mass Index (kg/m 2 ) | Blood Pressure (mm Hg) | HR at Rest (beats/min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 13 | 6 Months | Baseline | Week 13 | 6 Months | Baseline | Week 13 | 6 Months | ||||
| ET 1, family 1 | 59 | G2337V 46 RyR2 | Nadolol (40 mg/day) | 23.8 | 23.6 | 23.3 | 104/66 | 124/64 | 117/63 | 46 | 46 | 45 |
| ET 2, family 1 | 33 | G2337V 46 RyR2 | Nadolol (120 mg/day) | 25.5 | 26.4 | 27.8 | 110/68 | 98/67 | 98/61 | 56 | 67 | 69 |
| ET 3, family 1 | 54 | G2337V 46 RyR2 | Metoprolol (150 mg/day) | 27.0 | 26.4 | 25.6 | 133/73 | 123/69 | 129/66 | 60 | 58 | 64 |
| ET 4, family 1 | 60 | G2337V 46 RyR2 | Metoprolol (150 mg/day) | 33.8 | 34.8 | 34.5 | 128/85 | 132/73 | 174/78 | 56 | 52 | 55 |
| ET 5, family 2 | 26 | R176Q 8 RyR2 | Nadolol (120 mg/day) | 24.7 | 25.4 | 25.6 | 109/58 | 85/57 | 89/56 | 46 | 39 | 43 |
| ET 6, family 2 | 48 | R176Q 8 RyR2 | Nadolol (120 mg/day) | 23.0 | 23.0 | 23.0 | 85/63 | 88/67 | 78/58 | 46 | 47 | 43 |
| Patient SED | Age (yrs) | Mutation | Medication (Dose) | Body Mass Index (kg/m 2 ) | Blood Pressure (mm Hg) | HR at Rest (beats/min) | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 13 | Baseline | Week 13 | Baseline | Week 13 | ||||
| SED 1, family 1 | 26 | G2337V 46 RyR2 | Nadolol (80 mg/day) | 23.5 | 23.5 | 124/81 | 103/75 | 55 | 53 |
| SED 2, family 3 | 62 | G4671V 97 RyR2 | Metoprolol (50 mg/day) | 21.1 | 21.5 | 137/93 | 107/79 | 69 | 62 |
| SED 3, family 1 | 44 | G2337V 46 RyR2 | Metoprolol (50 mg/day) | 27.8 | 28.7 | 103/77 | 108/79 | 60 | 60 |
| SED 4, family 1 | 43 | G2337V 46 RyR2 | Carvedilol (25 mg/day) | 20.6 | 21.8 | 121/85 | 126/85 | 63 | 72 |
| SED 5, family 1 | 24 | G2337V 46 RyR2 | Metoprolol (200 mg/day) | 21.1 | 22.8 | 118/79 | 123/82 | 66 | 69 |
| SED 6, family 1 | 24 | G2337V 46 RyR2 | Nadolol (80 mg daily) | n.a | n.a | 123/80 | 135/75 | 60 | 41 |
| SED 7, family 4 | 17 | G2337V 46 RyR2 | Metoprolol (150 mg daily) | 23.0 | n.a | n.a | 113/68 | 58 | 53 |
Thirty-six training sessions were organized. The ET patients completed 28 ± 3 training sessions each, that is, 78 ± 8% program completion. No adverse events occurred during the training sessions.
VO 2 max increased for all ET patients, with an average increase of 13 ± 3% from baseline to week 13 (17.9 ± 1.3 vs 20.2 ± 1.6 ml/kg/min, p <0.05; Figure 2 ). Average VO 2 max was unaltered throughout the study period for SED patients ( Figure 2 ). Plasma cholesterol in ET patients decreased by 10 ± 5% from baseline to week 13 (5.9 ± 0.4 to 5.4 ± 0.3 mmol/L, p <0.05).




