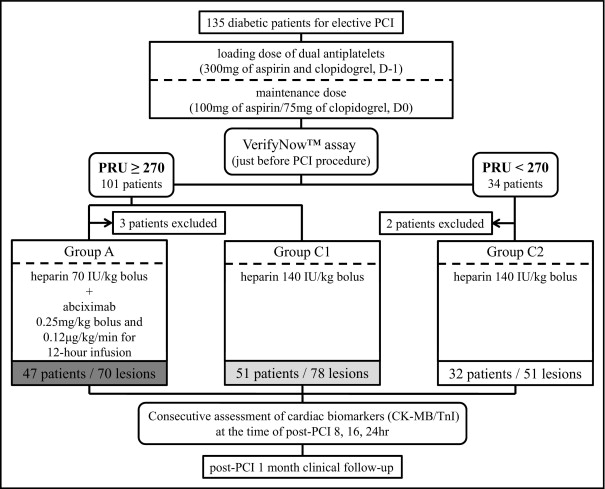
We investigated whether additional platelet inhibition with a glycoprotein IIb/IIIa inhibitor would be beneficial in reducing the risk of periprocedural myocardial infarction (PMI) in diabetic patients with high residual platelet reactivity (HPR). Patients with diabetes mellitus were administered aspirin and clopidogrel at a 300-mg loading dose 1 day before the procedure, and the VerifyNow P2Y 12 assay was performed just before percutaneous coronary intervention. Patients with HPR, defined as a P2Y 12 reaction unit of ≥270 were randomly assigned to group A or control group C1. Patients without HPR were assigned to control group C2. Conventional anticoagulation with heparin was given to groups C1 and C2, and group A received additional abciximab treatment. Clinically relevant PMI was defined as any elevation in the biomarkers creatine kinase-MB isoenzyme and cardiac troponin I >3 times the upper normal limit measured 8, 16, or 24 hours after percutaneous coronary intervention. Of the patients, 47 and 51 were assigned to group A and C1; the clinical and procedural characteristics in the 2 groups were balanced. Of the 47 patients in group A and 51 patients in group C1, 9 (19%) and 9 (18%), respectively, experienced a PMI event according to the creatine kinase-MB cutoff (p = 1.00), and 27 in group A (57%) and 29 in group C1 (57%) experienced a PMI event according to the troponin I cutoff (p = 1.00). Five minor bleeding events, including small and localized hematomas, were observed immediately after the procedure (4 in group A and 1 in group C1). Only 1 major bleeding event, retroperitoneal hemorrhage, was observed in group A. The patients in group C2 had a PMI event rate (50% of 32 patients, p = 1.00) similar to that of group C1. In conclusion, additional platelet inhibition using a tailored approach and a point-of-care assay did not improve the periprocedural outcome in diabetic patients with HPR.
High residual platelet reactivity (HPR) that persists despite antiplatelet therapy has been linked to impaired periprocedural outcomes in previous trials. These studies suggested that HPR might play a critical role in the development of periprocedural myocardial infarction (PMI). In patients with diabetes mellitus (DM), insufficient inhibition of platelet aggregation at the procedure can be more detrimental, even when applied with elective coronary stenting. The evidence from laboratory measurements has indicated that HPR reflects substantial interindividual variability in the platelet response to clopidogrel. These observations have provided the impetus for the use of point-of-care platelet function assays to identify at-risk patients and to predict their prognosis. We hypothesized that additional platelet inhibition with a glycoprotein IIb/IIIa inhibitor might be beneficial in reducing the risk of PMI in diabetic patients with HPR assessed using the VerifyNow P2Y 12 assay.
Methods
We prospectively enrolled diabetic patients who were candidates for planned percutaneous coronary intervention (PCI) from June 2009 to July 2011 at Seoul National University Bundang Hospital (Seoul, Korea). All patients recruited in the present study were enrolled consecutively after verification by negative cardiac biomarker study findings. The levels of the creatinine kinase-MB isoenzyme (Dimension Vista 1500 System, Siemens Healthcare Diagnostics, Munich, Germany) and cardiac troponin I (TnI; VITROS 5600 System, Ortho Clinical Diagnostics, Raritan, New Jersey) were measured simultaneously at admission. Patients were excluded if they had a history of allergy to, or intolerance of, aspirin or clopidogrel, a history of cerebrovascular or other major bleeding, major surgery within the previous 6 months, a history of acute myocardial infarction or ischemic cerebral infarction, platelet count <100,000/mm 3 , or hematocrit <30%. All patients provided written informed consent, and the local ethics review board reviewed the study scheme. The full protocol of the present study has been registered at http://www.clinicaltrials.gov (clinical trial no. NCT01475552 ).
All patients had DM and were treated routinely with both aspirin and clopidogrel at a 300-mg oral loading dose 1 day before the procedure. They were administered maintenance doses of 100 mg/day aspirin and 75 mg/day clopidogrel afterward. The VerifyNow P2Y 12 assay was performed just before PCI in patients who were scheduled to receive a coronary stent implant after diagnostic angiography. The detailed characteristics of this assay and its reliability have been previously reported. The results were reported as P2Y 12 reaction units and the percentage of inhibition. The cutoff value for HPR was P2Y 12 reaction units of ≥270, a value associated with atherothrombotic complications within 6 months after coronary stenting in Korean patients. After 1:1 randomization of patients with HPR, these patients were randomized further to group A or group C1, and patients without HPR were allocated to group C2. All patients were followed up for 1 month ( Figure 1 ). Groups C1 and C2 received conventional anticoagulation (140 U/kg intravenous bolus infusion) and group A was treated with a reduced dose of heparin (70 U/kg intravenous bolus infusion) and abciximab (0.25 mg/kg intravenous bolus followed by 0.125 mg/kg/hour continuous infusion for 12 hours). All procedures were performed in accordance with contemporary standards and at the physician’s discretion.
The primary objective of the present trial was to compare the prevalence of PMI, as reflected by the TnI concentration, between groups C1 and A. To evaluate the extent of myocardial damage during PCI, the TnI and creatine kinase-MB concentrations were both measured 8, 16, and 24 hours after PCI. In these successive assays, any increase in the level of these cardiac biomarkers greater than the 99th percentile upper limit of normal was defined as myonecrosis, and clinically relevant PMI was defined as a more than threefold elevation greater than the upper limit of normal for either of the 2 biomarkers. The laboratory upper limit of normal in our institution was 0.045 ng/ml for TnI and 0.28 ng/ml for creatine kinase-MB. The primary end point was the prevalence of PMI, as assessed by the TnI concentration. The secondary end point was the prevalence of PMI, as assessed by the creatine kinase-MB concentration. A greater than fivefold increase over the upper limit of normal for these biomarkers and the maximal extent of the change from the baseline value were also evaluated as secondary end points. Clinical events such as bleeding or major adverse cardiac events, defined as the composite of cardiac death, nonfatal spontaneous myocardial infarction, and urgent target vessel revascularization, were also noted at the 1-month follow-up visit. Bleeding events of safety concern were reported according to the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) bleeding classification. Quantitative coronary angiography was performed for all target lesions, and immediate post-PCI angiographic complications were analyzed for all cases. All biochemical, clinical, and angiographic findings were assessed in a blinded manner.
Assuming PMI event rates by the TnI criterion of 57% in group C1 and 29% in group A and a withdrawal rate of 5%, we estimated that a sample of 100 patients with P2Y 12 reaction units of ≥270 would provide 80% power to detect a 50% reduction in the PMI rate at the 2-sided α of 0.05. Categorical variables are presented as percentages, and the values were compared between groups using the chi-square or Fisher’s exact test. Continuous variables are presented as the mean ± SD and were compared between groups using an unpaired t test or Mann-Whitney U test. Nonparametric tests were used after confirming a non-normal distribution with the Kolmogorov-Smirnov test. p Values <0.05 were considered significant. The statistical analyses were performed using the SPSS, version 17.0, statistical package (IBM, New York, New York).
Results
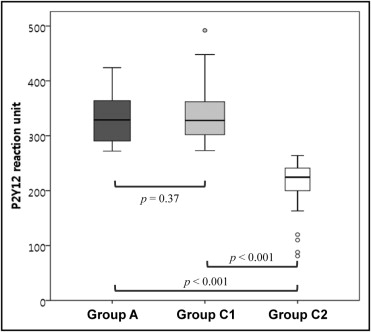
Figure 1 shows the study flow throughout the study period. Three patients in group A and 2 patients in group C2 withdrew because of failed PCI or withdrawal of consent. No differences were found in the P2Y 12 reaction units between groups A and C1 ( Figure 2 ). No bailout abciximab treatment was performed in group C1 or C2. The baseline clinical and angiographic characteristics did not differ between groups A and C1 ( Tables 1 and 2 ).
| Variable | PRU ≥270 | p Value (A vs C1) | PRU <270 Group C2 | p Value (C1 vs C2) | |
|---|---|---|---|---|---|
| Group A | Group C1 | ||||
| Demographic characteristics | |||||
| Men (%) | 28 (60%) | 27 (53%) | 0.509 | 24 (75%) | 0.064 |
| Age (years) | 67.5 ± 6.6 | 66.5 ± 7.2 | 0.555 | 64.4 ± 9.6 | 0.423 |
| Body mass index (kg/m 2 ) | 24.9 ± 2.5 | 25.6 ± 3.5 | 0.403 | 25.7 ± 2.6 | 0.603 |
| Clinical presentation | 0.079 | 0.001 | |||
| Stable angina | 35 (75%) | 45 (88%) | 18 (56%) | ||
| Unstable angina | 12 (26%) | 6 (12%) | 14 (44%) | ||
| Involved vessel | 0.425 | 0.922 | |||
| 1-Vessel disease | 14 (30%) | 13 (26%) | 9 (28%) | ||
| 2-Vessel disease | 17 (36%) | 25 (49%) | 16 (50%) | ||
| 3-Vessel disease | 16 (34%) | 13 (26%) | 7 (22%) | ||
| Left main disease | 5 (11%) | 7 (14%) | 0.641 | 6 (19%) | 0.540 |
| Diabetes duration (years) | 0.114 | 0.675 | |||
| Median | 10 | 10 | 9 | ||
| Interquartile range | 7–20 | 5–16 | 3–20 | ||
| Hemoglobin A1c (%) | 7.8 ± 1.6 | 7.7 ± 1.2 | 0.889 | 7.4 ± 1.3 | 0.308 |
| Chronic renal failure ⁎ | 11 (23%) | 10 (20%) | 0.647 | 4 (13%) | 0.440 |
| Hypertension | 42 (89%) | 46 (90%) | 1.000 | 30 (94%) | 0.701 |
| Dyslipidemia | 26 (55%) | 29 (57%) | 0.878 | 23 (72%) | 0.169 |
| Medications at admission | |||||
| Diabetes mellitus medications (n) | 0.171 | 0.091 | |||
| Median | 2 | 2 | 1 | ||
| Interquartile range | 1–2 | 1–2 | 1–2 | ||
| Insulin therapy | 13 (28%) | 9 (18%) | 0.235 | 6 (19%) | 0.899 |
| Antiplatelet agents | 0.852 | 0.395 | |||
| Naive | 7 (15%) | 10 (20%) | 4 (13%) | ||
| Single agents | 28 (60%) | 29 (57%) | 16 (50%) | ||
| Dual agents | 12 (26%) | 12 (24%) | 12 (38%) | ||
| Aspirin | 37 (79%) | 37 (73%) | 0.478 | 26 (81%) | 0.367 |
| Clopidogrel | 12 (26%) | 14 (28%) | 0.830 | 13 (41%) | 0.212 |
| Statin | 25 (53%) | 28 (55%) | 0.865 | 18 (56%) | 0.904 |
| Major laboratory findings | |||||
| Hemoglobin (g/dl) | 12.7 ± 1.4 | 13.2 ± 1.3 | 0.061 | 14.2 ± 1.8 | 0.007 |
| Platelet (×10 3 /μl) | 222 ± 54 | 224 ± 61 | 0.887 | 232 ± 65 | 0.575 |
| Activated partial thromboplastin time (s) | 35 ± 4 | 37 ± 7 | 0.105 | 35 ± 4 | 0.410 |
| Prothrombin time and international normalized ratio | 1.00 ± 0.07 | 1.00 ± 0.24 | 0.896 | 0.98 ± 0.08 | 0.632 |
| Fasting blood glucose (mg/dl) | 138 ± 60 | 119 ± 36 | 0.074 | 118 ± 40 | 0.868 |
| Total cholesterol (mg/dl) | 163 ± 40 | 161 ± 35 | 0.802 | 166 ± 41 | 0.565 |
| Low-density lipoprotein cholesterol (mg/dl) | 87 ± 29 | 90 ± 29 | 0.613 | 92 ± 34 | 0.806 |
| High-sensitivity C-reactive protein (mg/L) | 0.479 | 0.340 | |||
| Median | 3.0 | 3.0 | 3.0 | ||
| Interquartile range | 3.0–3.0 | 3.0–3.2 | 3.0–3.0 | ||
| Serum creatinine (mg/dl) | 1.06 ± 0.61 | 1.11 ± 1.39 | 0.819 | 0.94 ± 0.25 | 0.496 |
| Ejection fraction (%) | 61.1 ± 6.7 | 59.4 ± 7.9 | 0.267 | 57.3 ± 9.2 | 0.307 |
| P2Y 12 reaction units | 330 ± 40 | 336 ± 47 | 0.370 | 206 ± 58 | <0.001 |
| Percentage of inhibition of P2Y 12 reaction units | 11.5 ± 9.3 | 8.4 ± 8.9 | 0.091 | 34.6 ± 19.6 | <0.001 |
⁎ Estimated glomerular filtration rate <60 ml/min/1.73 m 2 .
| Variable | PRU ≥270 | p Value (A vs C1) | PRU <270 (Group C2) | p Value (C1 vs C2) | |
|---|---|---|---|---|---|
| Group A | Group C1 | ||||
| Lesion characteristics | |||||
| Target lesion | 0.50 | 0.63 | |||
| Left anterior descending artery | 31 (44%) | 42 (5%) | 23 (45%) | ||
| Left circumflex artery | 16 (23%) | 16 (21%) | 13 (26%) | ||
| Right coronary artery | 23 (33%) | 20 (26%) | 15 (29%) | ||
| Type B2/C lesion | 62 (89%) | 60 (71%) | 0.19 | 44 (86%) | 0.17 |
| De novo lesion | 69 (99%) | 75 (96%) | 0.62 | 49 (96%) | 1.00 |
| Chronic total occlusion | 5 (7%) | 3 (4%) | 0.48 | 7 (14%) | 0.05 |
| Ostial lesion | 3 (4%) | 1 (1%) | 0.34 | 6 (12%) | 0.02 |
| Bifurcation | 10 (14%) | 21 (27%) | 0.07 | 14 (28%) | 1.00 |
| Procedural characteristics | |||||
| Stents per target lesion (n) | 0.14 | 0.53 | |||
| Median | 1 | 1 | 1 | ||
| Interquartile range | 1–2 | 1–1 | 1–1 | ||
| Stent type | 1.00 | 0.87 | |||
| Plain balloon angioplasty | 4 (4%) | 4 (4%) | 2 (3%) | ||
| Bare metal stent | 2 (2%) | 2 (2%) | 2 (3%) | ||
| Drug-eluting stent | 86 (94%) | 87 (94%) | 59 (94%) | ||
| Intravascular ultrasound guidance | 14 (20%) | 21 (27%) | 0.32 | 11 (22%) | 0.49 |
| Direct stenting | 1 (1%) | 2 (3%) | 1.00 | 3 (6%) | 0.38 |
| Adjuvant ballooning | 55 (79%) | 63 (81%) | 0.84 | 41 (80%) | 1.00 |
| Maximal ballooning pressure (atm) | 0.11 | 0.22 | |||
| Median | 18 | 14 | 16 | ||
| Interquartile range | 12–20 | 12–18 | 14–20 | ||
| Femoral access | 42 (89%) | 44 (86%) | 0.76 | 27 (84%) | 1.00 |
| Anticoagulation after percutaneous coronary intervention | 1 (2%) | 3 (6%) | 0.62 | 0 (0%) | 0.28 |
| Quantitative coronary angiography | |||||
| Stent diameter (mm) | 0.19 | 0.09 | |||
| Median | 3.0 | 3.0 | 3.0 | ||
| Interquartile range | 2.5–3.0 | 2.8–3.3 | 2.8–3.5 | ||
| Total stent length (mm) | 0.80 | 0.91 | |||
| Median | 26 | 27 | 25 | ||
| Interquartile range | 18–48 | 22–34 | 18–38 | ||
| Before percutaneous coronary intervention | |||||
| Lesion length (mm) | 0.90 | 0.24 | |||
| Median | 21.8 | 23.5 | 21.7 | ||
| Interquartile range | 15.7–35.6 | 17.0–30.2 | 15.4–28.8 | ||
| Lesion reference diameter (mm) | 0.98 | 0.02 | |||
| Median | 2.8 | 2.8 | 3.0 | ||
| Interquartile range | 2.4–3.1 | 2.5–3.0 | 2.5–3.2 | ||
| Minimum lesion diameter (mm) | 0.06 | 0.87 | |||
| Median | 0.8 | 0.9 | 0.9 | ||
| Interquartile range | 0.5–1.1 | 0.7–1.2 | 0.60–1.1 | ||
| Lesion diameter stenosis (%) | 0.06 | 0.13 | |||
| Median | 72.5 | 67.1 | 71.2 | ||
| Interquartile range | 61.3–82.9 | 59.1–74.2 | 62.7–76.9 | ||
| After percutaneous coronary intervention | |||||
| Minimum lesion diameter (mm) | 0.11 | 0.06 | |||
| Median | 2.4 | 2.5 | 2.6 | ||
| Interquartile range | 2.1–2.7 | 2.2–2.8 | 2.3–3.0 | ||
| Lesion diameter stenosis (%) | 0.10 | 0.92 | |||
| Median | 12.6 | 10.7 | 11.8 | ||
| Interquartile range | 8.3–16.6 | 6.8–15.5 | 6.4–14.7 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


