The aim of our study was to compare the effect of simvastatin and fenofibrate treatment on the secretory function of human monocytes and lymphocytes and on systemic inflammation in type 2 diabetes and to assess whether their coadministration is superior to treatment with only 1 of these drugs. One hundred ninety-six adult patients with recently diagnosed and previously untreated type 2 diabetes and mixed dyslipidemia, complying throughout the study with lifestyle intervention and treated with metformin, were randomized in a double-blind fashion to receive simvastatin (40 mg), fenofibrate (200 mg), simvastatin plus fenofibrate, or placebo for 90 days. Main outcome measurements were monocyte and lymphocyte release of proinflammatory cytokines and plasma levels of C-reactive protein. One hundred ninety patients completed the study. Simvastatin and fenofibrate decreased monocyte release of tumor necrosis factor-α, interleukin-1β, interleukin-6, and monocyte chemoattractant protein-1 and lymphocyte release of interleukin-2, interferon-γ, and tumor necrosis factor-α, which was accompanied by a decrease in plasma C-reactive protein levels. Anti-inflammatory effects of fenofibrate partly correlated with the improvement in insulin sensitivity. Lymphocyte-suppressing, but not monocyte-suppressing, effect was stronger if these 2 agents were administered together. In conclusion, simvastatin and fenofibrate exhibit a similar effect on the secretory function of human monocytes and lymphocytes and on systemic inflammation in type 2 diabetic subjects with mixed dyslipidemia. This effect may be clinically relevant in the prevention of vascular complications in metformin- and diet-treated subjects with newly diagnosed diabetic dyslipidemia.
Recently we found that fenofibrate, particularly administered with metformin, produced pluripotential pleiotropic effects including a decrease in systemic inflammation and monocyte cytokine release and an improvement in insulin sensitivity and hemostasis in type 2 diabetic patients with mixed dyslipidemia. In this prospective, double-blind, placebo-controlled randomized study we have compared the effect of fenofibrate and simvastatin, used alone or in combination, on monocyte and lymphocyte secretory functions and systemic inflammation in type 2 diabetic patients with mixed dyslipidemia complying with lifestyle modification and treated with metformin. Monocytes/macrophages and lymphocytes are crucial cells involved in the development and progression of atherosclerosis and are present in large amounts in atherosclerotic plaque. Tumor necrosis factor-α (TNF-α), interleukin-1β, interleukin-6, monocyte chemoattractant protein-1 (MCP-1), interleukin-2, and interferon-γ were selected of the many monocyte- and lymphocyte-derived cytokines because these cytokines produce a multidirectional proatherogenic effect and our team has long-term experience in their assessment. C-reactive protein (CRP) has been identified as a sensitive marker of low-grade systemic inflammation of high prognostic value for determining cardiovascular risk and is directly involved in the initiation and progression of atherosclerosis.
Methods
Patients (25 to 75 years old) were eligible for the study if they met the following criteria: (1) recently diagnosed and previously untreated type 2 diabetes mellitus (fasting plasma glucose ≥126 mg/dl or plasma glucose concentration 2 hours after a glucose load ≥200 mg/dl) and (2) mixed dyslipidemia (plasma total cholesterol >200 mg/dl, low-density lipoprotein [LDL] cholesterol >130 mg/dl, triglycerides >150 mg/dl). Exclusion criteria are presented in the Supplemental Data (available online).
The study was performed in accordance with the Declaration of Helsinki and was approved by the local ethics committee. All patients gave written informed consent after the nature of the study had been explained. All included patients (n = 196) were given detailed advice about how to achieve the goals of lifestyle modification, which were a decrease in weight ≥7% if necessary, total fat intake <30% of total energy intake, saturated fat intake <7% of energy consumed, cholesterol intake <200 mg/day, an increase in fiber intake to 15 g/1,000 kcal, and moderate to vigorous exercise for ≥30 minutes/day. Average dietary adherence was assessed by food-frequency questionnaire and analysis of 3-day eating diaries by validated methods at every visit. Apart from dietary recommendations patients were prescribed with metformin, which was administered at a dose of 850 mg 1 times/day for the first week and thereafter 2 times/day for 90 days ( Figure 1 ). After 90 days of lifestyle intervention and metformin treatment, patients were randomized in double-blind fashion to 1 of 4 treatment groups that was treated with simvastatin (40 mg/day), fenofibrate (200 mg/day), simvastatin (40 mg/day) in combination with fenofibrate (200 mg/day), or placebo, respectively. For fenofibrate, a micronized form was used, which is more effective and convenient than its immediate-acting form. Simvastatin and fenofibrate were administered for 90 days without any changes in dosage during the entire study period. To minimize the risk of eventual pharmacokinetic interactions between simvastatin and fenofibrate, the 2 drugs were administered in 12-hour intervals (from 8:00 to 9:00 a . m . and from 8:00 to 9:00 p . m .). In the groups treated solely with simvastatin or fenofibrate, 1/2 of patients received a drug in the morning and placebo in the evening, and the other 1/2 was given the drug in the evening and placebo in the morning. In the combined therapy group, 1/2 of patients were treated with simvastatin in the morning and fenofibrate in the evening, and in the other 1/2 these drugs were administered in reverse order. Placebo-treated patients received placebo 2 times/day. Throughout the study period all patients complied with lifestyle modifications and were treated with metformin. Investigation of possible drug-induced side effects was performed fortnightly. Safety end points were overt myopathy, increase of aminotransferases >3 times the upper limit of normal, and creatine kinase levels >10 times the upper limit of normal. Compliance was assessed during each visit by tablet counts and was considered satisfactory when the number of tablets taken by a patient was 90% to 110%.
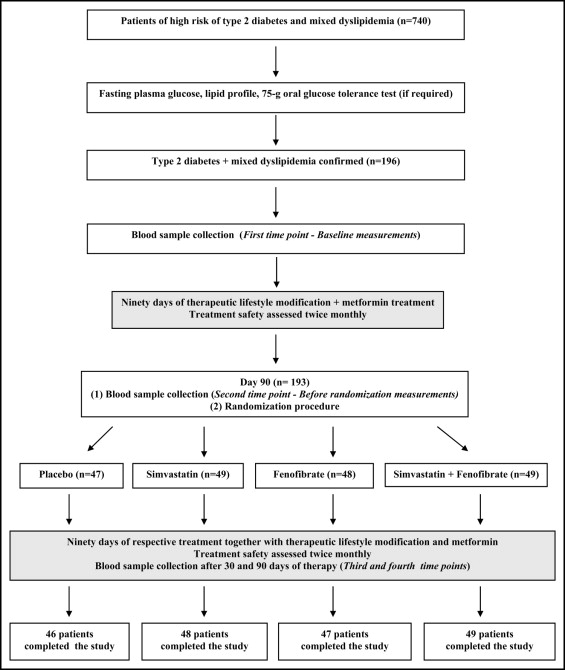
All measurements were performed 4 times: at baseline, before randomization, and after 30 and 90 days of therapy. Venous blood samples were taken 12 hours after the last meal in a quiet temperature-controlled room (24°C to 25°C) in constant daily hours (from 8:00 to 9:00 a . m .) to avoid circadian fluctuations of the parameters studied. Plasma levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were assessed colorimetrically using commercially available kits obtained from bioMérieux (Marcy l’Etoile, France). LDL cholesterol levels were measured directly. Plasma glucose concentrations were measured by a glucose oxidase method (Beckman, Palo Alto, California). Total nonesterified free fatty acids were determined by an enzymatic assay using reagents from Alpha Laboratories (Eastleigh, Hants, United Kingdom). Levels of apoproteins A-I and B were assessed by immunoturbidimetry (Incstar, Corp., Stillwater, Minnesota). Glycated hemoglobin was determined using DCA (2000) analyzer (Bayer, Ames Technicon, Tarrytown, New York). Plasma insulin was measured with a commercial radioimmunoassay kit (Linco Research, Inc., St. Charles, Missouri) that does not cross-react with human proinsulin. Homeostatic model assessment index was calculated as the product of fasting plasma insulin level (microunits per milliliter) and fasting plasma glucose level (millimoles per liter) divided by 22.5. Plasma levels of CRP were measured using a high-sensitivity monoclonal antibody assay (high-sensitivity CRP [hs-CRP]; MP Biomedicals, Orangeburg, New York). Lower limit of sensitivity of this method was 0.1 mg/L.
Cultures of phytohemagglutinin-stimulated T cells and lipopolysaccharide-stimulated monocytes were performed in triplicate as described previously. Monocyte release of TNF-α, interleukin-1β, interleukin-6, and MCP-1 and lymphocyte release of interleukin-2, interferon-γ, and TNF-α were estimated using commercial enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota) according to the manufacturer’s instructions. Minimum detectable levels for assessed cytokines were 8, 15, 4.4, 5.0, 3.9, and 1.0 pg/ml for interleukin-2, interferon-γ, TNF-α, MCP-1, interleukin-6, TNF-α, and interleukin-1β, respectively. Intra- and interassay coefficients of variation were <6.0% and <8.5%, respectively.
All calculations were made using GraphPad Prism 2.01 (GraphPad Software, Inc., San Diego, California) and Statistica 6.1 (StatSoft, Tulsa, Oklahoma). Statistical significance was defined as a p value <0.05. To begin with, distribution of variables was analyzed using Kolmogorov–Smirnov test. Outcomes for insulin, homeostatic model assessment index, hs-CRP, and cytokines were natural log-transformed to satisfy assumptions of normality and equal variance. Because lipid/lipoprotein and carbohydrate and, after logarithmic transformation, other values were normally distributed, parametric statistics were used for analyses. Comparisons between groups were performed using 1-way analysis of variance followed by post hoc Newman–Keuls test. Differences between means of variables within the same treatment group were analyzed with Student’s paired t test. In addition, to verify the correctness of statistical analysis for insulin, homeostatic model assessment index, hs-CRP, and cytokines, their median values on the original scale were recalculated using nonparametric tests (Kruskal–Wallis test followed by Mann–Whitney U test and Wilcoxon matched-pair test). Because the results of nonparametric statistics did not differ from those obtained after using nonparametric tests, these are not shown. Because the results of this analysis are consistent with those obtained after using parametric tests, these are not discussed in the text. For categorical variables, chi-square test was used. Correlations were calculated using the Kendall τ test.
Results
There were no significant differences in age, weight, and gender between treatment groups of diabetic patients with mixed dyslipidemia. All groups were comparable in medical background and clinical and laboratory characteristics ( Table 1 ).
| Variable | Placebo | Simvastatin | Fenofibrate | Simvastatin + Fenofibrate |
|---|---|---|---|---|
| (n = 46) | (n = 48) | (n = 47) | (n = 49) | |
| Age (years) | 52.8 ± 2.0 | 53.2 ± 1.9 | 52.6 ± 2.3 | 53.4 ± 2.0 |
| Women | 41% | 44% | 43% | 45% |
| Smokers | 20% | 21% | 23% | 24% |
| Body mass index (kg/m 2 ) | 28.2 ± 1.4 | 28.8 ± 2.0 | 29.0 ± 2.2 | 28.6 ± 1.7 |
| Atherogenic dyslipidemia † | 87% (76%) | 83% (73%) | 85% (74%) | 82% (71%) |
| Total cholesterol (mg/dl) | 235 ± 4 | 240 ± 5 | 238 ± 4 | 242 ± 5 |
| Low-density lipoprotein cholesterol (mg/dl) | 155 ± 2 | 156 ± 3 | 153 ± 4 | 159 ± 4 |
| High-density lipoprotein cholesterol (mg/dl) | 37 ± 1 | 37 ± 1 | 38 ± 1 | 37 ± 1 |
| Triglycerides (mg/dl) | 231 ± 6 | 235 ± 6 | 226 ± 7 | 237 ± 8 |
| Apoprotein A-I (mg/dl) | 121 ± 7 | 118 ± 4 | 121 ± 5 | 117 ± 4 |
| Apoprotein B (mg/dl) | 157 ± 5 | 159 ± 5 | 157 ± 4 | 159 ± 4 |
| Free fatty acids (μmol/L) | 516 ± 39 | 502 ± 26 | 512 ± 38 | 508 ± 30 |
| Fasting glucose (mg/dl) | 173 ± 3 | 176 ± 4 | 180 ± 3 | 172 ± 4 |
| Homeostatic model assessment index | 12.1 ± 0.5 | 11.9 ± 0.5 | 12.0 ± 0.5 | 11.6 ± 0.6 |
| Glycated hemoglobin (%) | 8.1 ± 0.2 | 7.8 ± 0.2 | 7.9 ± 0.2 | 8.0 ± 0.1 |
| High-sensitivity C-reactive protein (mg/dl) | 3.2 ± 0.2 | 3.4 ± 0.3 | 3.1 ± 0.3 | 3.5 ± 0.2 |
| Monocyte tumor necrosis factor-α release (pg/ml) | 1,720 ± 40 | 1,810 ± 50 | 1,789 ± 48 | 1,825 ± 45 |
| Monocyte interleukin-1β release (pg/ml) | 143 ± 5 | 139 ± 5 | 141 ± 5 | 146 ± 6 |
| Monocyte interleukin-6 release (ng/ml) | 10.3 ± 0.4 | 10.7 ± 0.4 | 10.9 ± 0.3 | 10.4 ± 0.4 |
| Monocyte monocyte chemoattractant protein-1 release (ng/ml) | 21.3 ± 1.0 | 21.2 ± 0.8 | 22.0 ± 1.1 | 20.9 ± 0.8 |
| Lymphocyte interleukin-2 release (ng/ml) | 7.1 ± 0.2 | 7.0 ± 0.2 | 7.2 ± 0.3 | 7.5 ± 0.4 |
| Lymphocyte interferon-γ release (ng/ml) | 68.2 ± 3.2 | 67.6 ± 5.0 | 67.1 ± 4.1 | 69.0 ± 6.0 |
| Lymphocyte tumor necrosis factor-α release (pg/ml) | 405 ± 19 | 413 ± 19 | 410 ± 20 | 416 ± 17 |
⁎ Only data of subjects who completed the study were included in the final analyses.
† Based on National Cholesterol Education Program Adult Treatment Panel III criteria (criteria proposed by Grundy and Small ).
Three patients terminated the study before randomization because of metformin-induced diarrhea, nausea, and increased flatulence. One subject treated with simvastatin was withdrawn from the study because of increased creatine kinase activity. Two subjects dropped out because of noncompliance with the study protocol. Neither significant adverse effects nor any complications were reported throughout the study period in the remaining participants. Baseline characteristics of the 6 subjects who were withdrawn from the study did not differ from the 190 completing the study (data not shown).
Thirty days of lifestyle modification and metformin treatment significantly decreased fasting plasma glucose, homeostatic model assessment index, and glycated hemoglobin but produced no effect on plasma lipid/lipoproteins, hs-CRP, and cytokine release ( Table 2 ). Continuation of lifestyle modification and metformin treatment in addition placebo for the next 90 days, apart from changes in plasma glucose, homeostatic model assessment index, and glycated hemoglobin, decreased also triglyceride levels and inhibited monocyte TNF-α and interleukin-6 release. However, it did not affect monocyte release of interleukin-1β and MCP-1 and lymphocyte release of interleukin-2, interferon-γ, and TNF-α. Simvastatin administered to diabetic patients with mixed dyslipidemia complying with lifestyle modification and treated with metformin decreased total cholesterol, LDL cholesterol, triglycerides, apoprotein B, and free fatty acids and increased HDL cholesterol and apoprotein A. Simvastatin did not change fasting glucose levels, glycated hemoglobin, and homeostatic model assessment index. Moreover, simvastatin treatment inhibited plasma hs-CRP, monocyte release of TNF-α, interleukin-1β, interleukin-6, and MCP-1 and lymphocyte release of interleukin-2, interferon-γ, and TNF-α. Simvastatin action on free fatty acids, hs-CRP, and cytokine release was stronger after 90 than after 30 days of administration. Fenofibrate treatment led to a decrease in total and LDL cholesterol, triglycerides, free fatty acids, fasting plasma glucose, homeostatic model assessment index and glycated hemoglobin , hs-CRP, and monocyte and lymphocyte cytokine release. It also increased HDL cholesterol and apoprotein A-I levels. Effect of fenofibrate on free fatty acids, homeostatic model assessment index, hs-CRP, and cytokine release was more pronounced after 90 than after 30 days of treatment. Combined treatment with simvastatin and fenofibrate decreased total and LDL cholesterol, triglycerides, apoprotein B, fasting plasma glucose, homeostatic model assessment index, and glycated hemoglobin , and increased HDL cholesterol and apoprotein A-I levels. Simvastatin administered with fenofibrate also decreased hs-CRP and inhibited monocyte release of TNF-α, interleukin-1β, interleukin-6, and MCP-1 and lymphocyte release of interleukin-2, interferon-γ, and TNF-α. Effect of simvastatin and fenofibrate on free fatty acids, homeostatic model assessment index, and cytokine release was stronger after 90 than after 30 days of treatment.
| Treatment Group | ||||
|---|---|---|---|---|
| Placebo | Simvastatin | Fenofibrate | Simvastatin + Fenofibrate | |
| (n = 46) | (n = 48) | (n = 47) | (n = 49) | |
| Total cholesterol (mg/dl) | ||||
| Baseline | 235 ± 4 | 240 ± 5 | 238 ± 4 | 242 ± 5 |
| Before randomization | 233 ± 4 (−1) | 235 ± 5 (−2) | 229 ± 4 (−4) | 236 ± 5 (−3) |
| After 30 d of hypolipidemic treatment | 231 ± 4 (−2, −1) | 178 ± 4 (−26, −24) ‡ ∥ ‡‡‡ | 201 ± 4 (−16, −12) ⁎ § | 168 ± 4 (−31, −29) ‡ ¶ §§§ |
| After 90 days of hypolipidemic treatment | 230 ± 5 (−2, −2) | 177 ± 4 (−26, −25) ‡ ¶ §§§ | 201 ± 3 (−16, −12) † § # | 156 ± 5 (−36, −34) ‡ ¶ # §§§ |
| Low-density lipoprotein cholesterol (mg/dl) | ||||
| Baseline | 155 ± 2 | 156 ± 3 | 153 ± 4 | 159 ± 4 |
| Before randomization | 151 ± 3 (−3) | 152 ± 3 (−3) ⁎⁎ | 148 ± 3 (−4) | 155 ± 4 (−3) |
| After 30 days of hypolipidemic treatment | 151 ± 3 (−3.0) | 104 ± 3 (−33, −31) ‡ †† ¶ ††† | 122 ± 3 (−20, −17) ⁎ § | 96 ± 3 (−39, −38) ‡ ¶ †† ‡‡‡ |
| After 90 days of hypolipidemic treatment | 148 ± 5 (−5, −2) | 100 ± 2 (−36, −34) ‡ †† ¶ ††† | 120 ± 4 (−22, −19) ⁎ # § | 92 ± 3 (−42, −43) ‡ ¶ †† ‡‡‡ |
| High-density lipoprotein cholesterol (mg/dl) | ||||
| Baseline | 37 ± 1 | 37 ± 1 | 38 ± 1 | 37 ± 1 |
| Before randomization | 38 ± 1 (2) | 37 ± 1 (1.6) | 39 ± 1 (2) | 38 ± 1 (2) |
| After 30 days of hypolipidemic treatment | 39 ± 2 (5.3) | 42 ± 1 (15.13) ⁎ | 46 ± 1 (23.20) † § # | 47 ± 1 (28.25) † § †† |
| After 90 days of hypolipidemic treatment | 39 ± 1 (6.4) | 43 ± 1 (16.14) ⁎ | 48 ± 1 (27.25) ‡ ∥ †† | 50 ± 1 (36.33) ‡ ¶ †† ¶¶ |
| Triglycerides (mg/dl) | ||||
| Baseline | 231 ± 6 | 235 ± 6 | 226 ± 7 | 237 ± 8 |
| Before randomization | 208 ± 5 (−10) | 212 ± 6 (−10) | 204 ± 7 (−10) | 205 ± 9 (−13) |
| After 30 days of hypolipidemic treatment | 192 ± 5 (−17, −8) ⁎ | 179 ± 5 (−24, −16) ⁎ | 144 ± 8 (−36, −29) ‡ ¶ †† ## | 136 ± 6 (−43, −34) ‡ ¶ †† ⁎⁎ |
| After 90 days of hypolipidemic treatment | 191 ± 5 (−17, −9) ⁎ | 178 ± 5 (−24, −16) ⁎ | 141 ± 8 (−38, −32) ‡ ¶ †† ## | 123 ± 5 (−48, −40) ‡ ¶ †† ⁎⁎⁎ |
| Apoprotein A-I (mg/dl) | ||||
| Baseline | 121 ± 7 | 118 ± 4 | 121 ± 5 | 117 ± 4 |
| Before randomization | 130 ± 5 (7) | 127 ± 5 (8) | 132 ± 5 (10) | 128 ± 4 (9) |
| After 30 days of hypolipidemic treatment | 135 ± 5 (11.3) | 136 ± 4 (15.7) | 160 ± 5 (32.7, 21.0) ‡ § †† ## | 165 ± 4 (41.29) ‡ ∥ †† ## |
| After 90 days of hypolipidemic treatment | 136 ± 5 (12.5) | 143 ± 4 (21.13) ⁎ | 161 ± 3 (33.7, 21.8) ‡ § †† ¶¶ | 172 ± 3 (46.34) ‡ ∥ †† ## |
| Apoprotein B (mg/dl) | ||||
| Baseline | 157 ± 5 | 159 ± 5 | 157 ± 4 | 159 ± 4.3 |
| Before randomization | 150 ± 5 (−5) | 150 ± 4 (−6) | 152 ± 4 (−3) | 154 ± 4.0 (−3.5) |
| After 30 days of hypolipidemic treatment | 149 ± 5 (−6, −1) | 110 ± 3 (−31, −27) ‡ †† ¶ ††† | 130 ± 4 (−17, −14) † § # | 105 ± 3.5 (−33.9, −31.5) ‡ ¶ †† ‡‡‡ |
| After 90 days of hypolipidemic treatment | 148 ± 3 (−6, −1) | 108 ± 5 (−32, −28) ‡ ¶ †† ‡‡‡ | 130.2 ± 3 (−17, −14) † § # | 102 ± 3.8 (−35.8, −33.5) ‡ ¶ †† §§§ |
| Free fatty acids (μmol/L) | ||||
| Baseline | 516 ± 39 | 502 ± 26 | 512 ± 38 | 508 ± 30 |
| Before randomization | 438 ± 50 (−15) | 426 ± 39 (−15) | 435 ± 34 (−15) | 422 ± 42 (−17) |
| After 30 days of hypolipidemic treatment | 427 ± 40 (−17, −3) | 335 ± 33 (−33, −21) ‡ § # | 320 ± 37 (−38, −26) ‡ ∥ ⁎⁎ | 301 ± 21 (−41, −29) ‡ ¶ †† |
| After 90 days of hypolipidemic treatment | 420 ± 46 (−19, −4) | 265 ± 22 (−47, −38) ‡ ¶ ⁎⁎ ‡‡ | 250 ± 29 (−51, −43) ‡ ¶ †† ‡‡ | 220 ± 22 (−57, −48) ‡ ¶ †† §§ |
| Fasting glucose (mg/dl) | ||||
| Baseline | 173 ± 4 | 176 ± 4 | 180 ± 3 | 172 ± 4 |
| Before randomization | 144 ± 3 (−17) † | 147 ± 4 (−16) † | 142 ± 3 (−21) ‡ | 140 ± 2 (−18) ‡ |
| After 30 days of hypolipidemic treatment | 140 ± 2 (−19, −3) ‡ | 142 ± 3 (−18, −3) ‡ | 128 ± 2 (−29, −10) ‡ § ⁎⁎ ## | 126 ± 2 (−26, −10) ‡ ∥ ⁎⁎ ## |
| After 90 days of hypolipidemic treatment | 138 ± 3 (−20, −4) ‡ | 141 ± 3 (−19, −4) ‡ | 127 ± 3 (−30, −11) ‡ ∥ ⁎⁎ ## | 127 ± 3 (−26, −10) ‡ ∥ ⁎⁎ ## |
| Homeostatic model assessment | ||||
| Baseline | 12.1 ± 0.5 | 11.9 ± 0.5 | 12.0 ± 0.5 | 11.6 ± 0.6 |
| Before randomization | 10.9 ± 0.5 (−10) ⁎ | 10.5 ± 0.4 (−12) ⁎ | 10.7 ± 0.6 (−11) ⁎ | 10.2 ± 0.5 (−12) ⁎ |
| After 30 days of hypolipidemic treatment | 10.1 ± 0.6 (−17, −7) ⁎ | 9.8 ± 0.4 (−18, −7) † | 8.5 ± 0.4 (−29, −21) ‡ ⁎⁎ ¶¶ | 8.1 ± 0.3 (−30, −21) ‡ ∥ # ⁎⁎ ## |
| After 90 days of hypolipidemic treatment | 8.7 ± 0.5 (−28, −20) ‡ # | 9.2 ± 0.5 (−23, −12) † | 6.5 ± 0.4 (−46, −39) ‡ ∥ †† §§ ⁎⁎⁎ | 6.2 ± 0.4 (−47, −39) ‡ ∥ †† §§ ⁎⁎⁎ |
| Glycated hemoglobin (%) | ||||
| Baseline | 8.1 ± 0.2 | 7.8 ± 0.2 | 7.9 ± 0.2 | 8.0 ± 0.1 |
| Before randomization | 7.6 ± 0.1 (−6) ⁎ | 7.0 ± 0.2 (−10) ⁎ | 7.1 ± 0.2 (−10) ⁎ | 7.0 ± 0.2 (−13) † |
| After 30 days of hypolipidemic treatment | 7.4 ± 0.2 (−9, −3) ⁎ | 6.9 ± 0.2 (−12, −1) ⁎ | 6.3 ± 0.3 (−20, −11) ‡ ∥ # | 6.3 ± 0.2 (−21, −10) ‡ ∥ # |
| After 90 days of hypolipidemic treatment | 7.4 ± 0.2 (−9, −3) ⁎ | 6.8 ± 0.1 (−13, −3) † | 6.1 ± 0.2 (−23, −14) ‡ ∥ # ¶¶ | 6.0 ± 0.3 (−25, −14) ‡ ¶ # ¶¶ |
| High-sensitivity C-reactive protein (mg/dl) | ||||
| Baseline | 3.2 ± 0.2 | 3.4 ± 0.3 | 3.1 ± 0.3 | 3.5 ± 0.2 |
| Before randomization | 3.0 ± 0.2 (−6) | 3.0 ± 0.3 (−12) | 2.8 ± 0.2 (−10) | 3.1 ± 0.2 (−11) |
| After 30 days of hypolipidemic treatment | 2.9 ± 0.3 (−9, −3) | 2.5 ± 0.2 (−27, −17) ‡ # | 2.2 ± 0.2 (−29, −21) ‡ ∥ # | 2.1 ± 0.2 (−40, −32) ‡ ∥ †† |
| After 90 days of hypolipidemic treatment | 2.8 ± 0.2 (−13, −7) | 1.7 ± 0.2 (−50, −43) ‡ ¶ †† §§ | 1.6 ± 0.2 (−48, −43) ‡ ¶ †† §§ | 1.1 ± 0.1 (−69, −65) ‡ ¶ †† ∥∥ ¶¶ ††† |
| Monocyte tumor necrosis factor-α release (pg/ml) | ||||
| Baseline | 1,720 ± 40 | 1,810 ± 50 | 1,789 ± 48 | 1,825 ± 45 |
| Before randomization | 1,580 ± 51 (−9) | 1,600 ± 50 (−12) | 1,586 ± 45 (−11) | 1,621 ± 42 (−11) |
| After 30 days of hypolipidemic treatment | 1,269 ± 40 (−26, −20) ⁎ | 1,213 ± 30 (−33, −24) ‡ ⁎⁎ | 1,240 ± 9 (−31, −22) † # | 1,185 ± 40 (−35, −27) ‡ ⁎⁎ |
| After 90 days of hypolipidemic treatment | 1,250 ± 53 (−27, −21) ⁎ | 874 ± 26 (−52, −45) ‡ # ∥ ⁎⁎ ‡‡ | 890 ± 33 (−50, −44) ‡ ∥ # ‡‡ | 793 ± 30 (−57, −51) ‡ ¶ †† ∥∥ |
| Monocyte interleukin-1β release (pg/ml) | ||||
| Baseline | 143 ± 5 | 139 ± 5 | 141 ± 5 | 146 ± 6 |
| Before randomization | 140 ± 5 (−1.7) | 130 ± 6 (−6) | 132 ± 6 (−6) | 132 ± 6 (−9) |
| After 30 days of hypolipidemic treatment | 135 ± 6 (−5, −4) | 91 ± 4 (−35, −31) ‡ ¶ ⁎⁎ | 93.8 ± 5 (−33, −29) ‡ ∥ ⁎⁎ | 94 ± 5 (−36, −29) ‡ ¶ ⁎⁎ |
| After 90 days of hypolipidemic treatment | 134 ± 6 (−6, −5) | 73 ± 4 (−47, −44) ‡ ¶ †† ‡‡ | 71.2 ± 3 (−49, −46) ‡ ¶ †† §§ | 71 ± 4 (−51, −46) ‡ ¶ †† §§ |
| Monocyte interleukin-6 release (ng/ml) | ||||
| Baseline | 10.3 ± 0.4 | 10.7 ± 0.4 | 10.9 ± 0.3 | 10.4 ± 0.4 |
| Before randomization | 9.2 ± 0.3 (−11) | 9.5 ± 0.3 (−12) | 9.7 ± 0.4 (−11) | 9.8 ± 0.4 (−6) |
| After 30 days of hypolipidemic treatment | 8.0 ± 0.3 (−22, −13) ⁎ | 7.6 ± 0.3 (−29, −20) ⁎ # | 7.7 ± 0.3 (−29, −21) ⁎ # | 7.3 ± 0.3 (−30, −26) † # |
| After 90 days of hypolipidemic treatment | 7.9 ± 0.4 (−23, −14) ⁎ | 5.8 ± 0.3 (−46, −39) ‡ ∥ # ⁎⁎ §§ | 6.0 ± 0.4 (−45, −38) ‡ ∥ # ⁎⁎ ‡‡ | 5.4 ± 0.4 (−48, −45) ‡ ¶ †† §§ |
| Monocyte monocyte chemoattractant protein-1 release (ng/ml) | ||||
| Baseline | 21.3 ± 1.0 | 21.2 ± 0.8 | 22.0 ± 1.1 | 20.9 ± 0.8 |
| Before randomization | 19.6 ± 1.1 (−8) | 20.4 ± 0.9 (−5) | 20.3 ± 0.8 (−8) | 19.4 ± 0.7 (−7) |
| After 30 days of hypolipidemic treatment | 19.0 ± 1.4 (−11, −3) | 17.1 ± 0.9 (−19, −16) ⁎ | 16.9 ± 0.9 (−23, −17) ⁎ # | 15.4 ± 0.6 (−26, −21) ⁎ # |
| After 90 days of hypolipidemic treatment | 19.2 ± 0.8 (−10, −2) | 13.1 ± 0.6 (−38, −36) ‡ ∥ # ‡‡ | 12.5 ± 0.7 (−43, −38) ‡ ∥ # ‡‡ | 11.3 ± 0.7 (−46, −42) ‡ ¶ †† §§ |
| Lymphocyte interleukin-2 release (ng/ml) | ||||
| Baseline | 7.1 ± 0.2 | 7.0 ± 0.2 | 7.2 ± 0.3 | 7.5 ± 0.4 |
| Before randomization | 6.8 ± 0.2 (−4) | 6.8 ± 0.3 (−3) | 6.7 ± 0.2 (−7) | 7.1 ± 0.3 (−5) |
| After 30 days of hypolipidemic treatment | 6.6 ± 0.1 (−7, −3) | 5.2 ± 0.2 (−26, −24) † § # | 5.0 ± 0.1 (−31, −25) † § # | 4.6 ± 0.2 (−39, −35) ‡ ∥ †† |
| After 90 days of hypolipidemic treatment | 6.4 ± 0.1 (−10, −6) | 4.0 ± 0.2 (−43, −41) ‡ ¶ ⁎⁎ ‡‡ | 3.8 ± 0.2 (−47, −43) ‡ ¶ †† §§ | 2.8 ± 0.2 (−63, −61) ‡ ¶ †† ∥∥ ⁎⁎⁎ §§§ |
| Lymphocyte interferon-γ release (ng/ml) | ||||
| Baseline | 68.2 ± 3.2 | 67.6 ± 5.0 | 67.1 ± 4.1 | 69.0 ± 6.0 |
| Before randomization | 63.7 ± 3.0 (−7) | 64.8 ± 5.1 (−4) | 63.2 ± 4.9 (−6) | 64.2 ± 5.3 (−7) |
| After 30 days of hypolipidemic treatment | 62.1 ± 4.0 (−9, −3) | 51.1 ± 4.4 (−24, −21) † # | 47.9 ± 4.0 (−29, −24) † ∥ ⁎⁎ | 42.8 ± 3.8 (−38, −33) ‡ ¶ †† |
| After 90 days of hypolipidemic treatment | 61.9 ± 3.5 (−9, −3) | 38.1 ± 3.5 (−44, −41) ‡ ¶ †† §§ | 37.5 ± 3.3 (−44, −41) ‡ ¶ †† ‡‡ | 28.6 ± 2.9 (−59, −55) ‡ ¶ †† §§ ## ††† |
| Tumor necrosis factor-α release (pg/ml) | ||||
| Baseline | 405 ± 12 | 413 ± 19 | 410 ± 20 | 416 ± 18 |
| Before randomization | 387 ± 18 (−4) | 398 ± 21 (−3) | 393 ± 19 (−4) | 398 ± 18 (−4) |
| After 30 days of hypolipidemic treatment | 361 ± 18 (−11, −7) | 310 ± 13 (−25, −22) † # | 316 ± 12 (−23, −20) † # | 298 ± 16 (−28, −26) ‡ § ⁎⁎ |
| After 90 days of hypolipidemic treatment | 359 ± 18 (−12, −7) | 246 ± 12 (−41, −38) ‡ ¶ †† ‡‡ | 251 ± 14 (−39, −36) ‡ ¶ †† ‡‡ | 179 ± 13 (−57, −56) ‡ ¶ †† ∥∥ ## §§§ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


