We used intravascular ultrasonography to assess plaque morphology and morphometry in 310 patients with acute myocardial infarction (125 with ST-segment elevation and 185 with non–ST-segment elevation myocardial infarction) with varying degrees of renal dysfunction according to the creatinine clearance (CrCl): CrCl >70 ml/min in 153, CrCl of 30 to 69 ml/min in 103, and CrCl of <30 ml/min in 54 patients, including 20 patients requiring dialysis). The lesion site plaque burden was greatest (77.4 ± 11.0% vs 79.8 ± 12.5% vs 82.0 ± 10.3%, p = 0.031) and the lesion was longest (20.9 ± 9.1 vs 23.1 ± 9.5 vs 26.3 ± 9.6 mm, p = 0.038) in the lowest CrCl group. Infarct-related artery plaque rupture (31.4% vs 34.0% vs 53.7%, p = 0.011) and multiple plaque ruptures (11.1% vs 12.6% vs 33.3%, p <0.001) were the most common, the ruptured plaque cavities were the largest (1.98 ± 0.89 vs 2.20 ± 1.45 vs 3.06 ± 1.70 mm 2 , p = 0.002), and the ruptured plaque was longest (2.33 ± 0.93 vs 2.59 ± 1.50 vs 3.33 ± 1.76 mm, p = 0.008) in the lowest CrCl group (<30 ml/min). Intravascular ultrasound-detected thrombus was observed most frequently in the lowest CrCl group (22.9% vs 23.3% vs 40.7%, p = 0.027). CrCl was the one of the independent predictors of culprit lesion plaque rupture (odds ratio 0.979, 95% confidence interval 0.963 to 0.994, p = 0.008). During 1 year of follow-up, the incidence of nonfatal myocardial infarction (2.6% vs 4.9% vs 11.1%, p = 0.044) and cardiac death (3.9% vs 6.8% vs 14.8%, p = 0.024) was greatest in the lowest CrCl group. Also, a strong trend was found toward the greatest incidence of stent thrombosis (2.0% vs 3.9% vs 9.3%, p = 0.057) in the lowest CrCl group. In conclusion, patients with acute myocardial infarction and significant renal dysfunction had more plaque vulnerability compared to those with normal renal function. This might be associated with poor clinical outcomes in patients with acute myocardial infarction and renal dysfunction.
Patients with acute myocardial infarction (AMI) have various vulnerable plaque characteristics on intravascular ultrasound (IVUS) examination. One IVUS study demonstrated that patients with end-stage renal disease had larger reference segment arterial and lumen areas; larger lesion site arterial, lumen, and plaque areas; larger arcs of calcium; and progressive calcific atherosclerosis. To date, no study has examined the plaque characteristics using IVUS studies in patients with AMI according to renal function. Therefore, the purpose of the present study was to assess plaque morphology and plaque morphometry using IVUS examination in patients with AMI and varying degrees of renal dysfunction.
Methods
The present study was a retrospective, single-center study. A total of 2,995 patients with a first AMI were admitted to our institute from August 2004 to July 2008. We performed preintervention IVUS examination of coronary culprit lesions in infarct-related arteries within 24 hours of symptom onset in 380 patients. Of these 380 patients, we excluded 5 patients with subacute or late stent thrombosis, 3 with coronary artery bypass graft failure, 32 with cardiogenic shock, 20 with important systemic disease, and 10 patients in whom adequate IVUS images could not be obtained. Thus, the study population consisted of 310 patients with AMI (125 with ST-segment elevation and 185 with non–ST-segment elevation myocardial infarction). The diagnosis of AMI was according to a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. The patients were divided into 3 groups according to the degree of renal function as determined by the calculated creatinine clearance (CrCl): CrCl >70 ml/min (n = 153); CrCl 30 to 69 ml/min (n = 103); and CrCl <30 ml/min (n = 54, including 20 patients requiring dialysis). The CrCl was calculated by applying the Cockcroft-Gault formula using the baseline serum creatinine level: CrCl = [(140 − age) × weight/serum creatinine × 72], with female gender adjustment (CrCl female = CrCl × 0.85). All 310 infarct lesions were treated with stent implantation: 138 with sirolimus-eluting stents (Cypher stent, Cordis, Johnson & Johnson, Miami Lakes, Florida), 49 with paclitaxel-eluting stents (Taxus stent, Boston Scientific, Boston, Massachusetts), and 123 with bare metal stents. The institutional review board approved the protocol.
The absolute creatine kinase-MB levels were determined by radioimmunoassay (Dade Behring, Miami, Florida). Cardiac-specific troponin I levels were measured by a paramagnetic particle, chemiluminescent immunoenzymatic assay (Beckman-Coulter, Fullerton, California). High-sensitivity C-reactive protein was assessed using the immunoturbidimetric C-reactive protein-Latex (II) high-sensitivity assay using an Olympus 5431 AutoAnalyzer (Denka Seiken, Tokyo, Japan). The assay was performed according to the manufacturer’s protocol and has been validated against the Dade-Behring method.
Coronary angiograms were analyzed using a validated quantitative coronary angiographic system (Phillips H5000 or Allura DCI program, Philips Medical Systems, Best, The Netherlands). With the outer diameter of the contrast-filled catheter as the calibration standard, the minimal lumen diameter, reference diameter, and lesion length were measured in diastolic frames from orthogonal projections. Perfusion was evaluated according to the Thrombolysis In Myocardial Infarction criteria. No-reflow was defined as Thrombolysis In Myocardial Infarction grade 0, 1, or 2 flow after percutaneous coronary intervention in the absence of mechanical obstruction. Normal reflow was defined as Thrombolysis In Myocardial Infarction grade 3 flow. If the Thrombolysis In Myocardial Infarction flow after percutaneous coronary intervention was 0, 1, or 2 in the absence of angiographic stenosis, a repeat IVUS examination was performed to exclude the possibility of mechanical vessel obstruction.
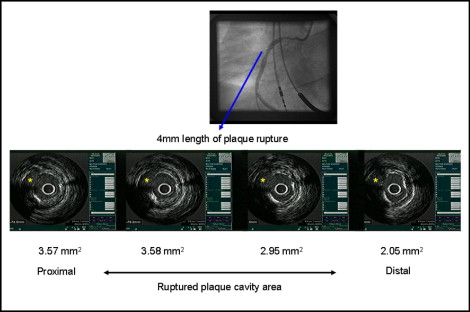
All IVUS examinations were performed before stenting using a commercially available IVUS system (Boston Scientific/SCIMed, Minneapolis, Minnesota). Qualitative analysis was performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies. Using planimetry software (Tape Measure, INDEC Systems, Mountain View, California), we measured the external elastic membrane (EEM) and lumen cross-sectional area (CSA). The plaque plus media CSA was calculated as the EEM CSA minus the lumen CSA, and the plaque burden was calculated as the plaque plus media CSA divided by the EEM CSA. The lesion was the site with the smallest lumen CSA. If multiple image slices showed the same minimum lumen CSA, the image slice with the largest EEM and plaque plus media was measured. A culprit lesion with ruptured plaque was defined as that containing a cavity that communicated with the lumen with an overlying residual fibrous cap fragment ( Figure 1 ). Rupture sites separated by a length of artery containing smooth lumen contours without cavities were considered to represent different plaque ruptures (infarct-related artery with multiple plaque ruptures). The identification of thrombus required ≥2 of the following: a distinct hypoechoic mass, brightly speckled plaque, channeling within the plaque, evacuated plaque cavity, or detached mobile mass. Soft plaque was less bright compared to the reference adventitia. Fibrotic plaque was as bright as, or brighter than, the reference adventitia without acoustic shadowing. Calcific plaque was hyperechoic with shadowing. A calcified lesion contained >90° of circumferential lesion calcium. When no dominant plaque composition was seen, the plaque was classified as mixed. Coronary artery remodeling was assessed by comparing the lesion site to the reference EEM CSA. The remodeling index was the lesion site EEM CSA divided by the average of the proximal and distal reference EEM CSA.
The hospital records of all the patients were reviewed to obtain information on the clinical demographics and medical history. Follow-up information was obtained through review of the hospital charts, telephone interviews, and the interventional database of the Heart Center of Chonnam National University Hospital (Gwangju, Korea). All deaths were considered of cardiac origin unless a noncardiac origin had been established clinically or at autopsy. Target lesion revascularization was defined as any intervention to treat in-stent restenosis, including stent edges within 5 mm proximal or distal to the stent. Nonfatal myocardial infarction was defined as ischemic symptoms associated with cardiac enzyme elevation of ≥3 times the upper limit of normal. Stent thrombosis included definite, probable, or possible stent thrombosis, classified according to the Academic Research Consortium definition.
The Statistical Package for Social Sciences for Windows, version 15.0 (SPSS, Chicago, Illinois) was used for all analyses. Continuous data are expressed as the mean ± SD and categorical data as frequencies and percentages. Differences in continuous variables were tested by analysis of variance for normal or log-normal distributed variables (ie, age, ejection fraction, and CrCl). The Kruskal-Wallis test was used for other variables (ie, creatine kinase-MB, cardiac-specific troponin I, and high-sensitivity C-reactive protein). Differences in the categorical variables were tested using the chi-square test or Fisher’s exact test. Pearson’s correlation coefficient was used to evaluate the associations between CrCl and various clinical and IVUS parameters. Multivariate analysis was performed to identify independent predictors of culprit lesion plaque rupture. A p value <0.05 was considered statistically significant.
Results
The baseline characteristics are summarized in Table 1 . The CrCl was 92.0 ± 21.1 mg/min in the highest CrCl group, 47.9 ± 8.7 mg/min in the middle CrCl group, and 16.1 ± 9.5 mg/min in lowest CrCl group. Patients with lowest CrCl were the oldest and had the greatest incidence of diabetes mellitus and hypertension. The left ventricular ejection fraction was lowest in the lowest CrCl group. The high-sensitivity C-reactive protein, troponin I, and white blood cell count levels were the greatest and the hemoglobin was the lowest in the lowest CrCl group.
| Variable | CrCl (ml/min) Group | p Value | ||
|---|---|---|---|---|
| ≥70 (n = 153) | 30–69 (n = 103) | <30 (n = 53) | ||
| Age (years) | 57 ± 12 | 62 ± 15 | 69 ± 11 | <0.001 |
| Men | 94 (61.4%) | 60 (58.3%) | 25 (46.3%) | 0.152 |
| Clinical presentation | 0.026 | |||
| ST-segment elevation myocardial infarction | 73 (47.7%) | 36 (35.0%) | 16 (29.6%) | |
| Non–ST-segment elevation myocardial infarction | 80 (52.3%) | 67 (65.0%) | 38 (70.4%) | |
| Diabetes mellitus | 36 (23.5%) | 38 (36.9%) | 37 (68.5%) | <0.001 |
| Hypertension | 97 (63.4%) | 78 (75.7%) | 43 (79.6%) | 0.027 |
| Smoking | 53 (34.6%) | 38 (36.9%) | 14 (25.9%) | 0.371 |
| Family history of coronary artery disease | 22 (14.4%) | 23 (22.3%) | 7 (13.0%) | 0.177 |
| Ejection fraction (%) | 47.2 ± 11.9 | 41.2 ± 13.4 | 40.9 ± 11.7 | <0.001 |
| Creatinine (mg/dl) | 0.92 ± 0.19 | 1.26 ± 0.30 | 4.86 ± 2.14 | <0.001 |
| Creatinine clearance (ml/min) | 92.0 ± 21.1 | 47.9 ± 8.7 | 16.1 ± 9.5 | <0.001 |
| High-sensitivity C-reactive protein (mg/L) | 17.5 ± 14.5 | 16.7 ± 11.2 | 43.4 ± 34.4 | 0.016 |
| Creatine kinase-MB (U/L) | 17.9 ± 24.8 | 24.8 ± 30.4 | 35.9 ± 40.0 | 0.182 |
| Cardiac-specific troponin I (ng/ml) | 11.5 ± 23.0 | 13.8 ± 24.5 | 28.1 ± 31.7 | 0.047 |
| White blood cell count (×1,000/mm 3 ) | 9.3 ± 3.5 | 8.3 ± 3.0 | 10.0 ± 4.0 | 0.023 |
| Hemoglobin (mg/dl) | 13.0 ± 1.6 | 12.3 ± 2.1 | 10.4 ± 2.4 | <0.001 |
| Platelet count (×1,000/mm 3 ) | 236 ± 72 | 220 ± 99 | 228 ± 93 | 0.506 |
| Total cholesterol (mg/dl) | 176 ± 44 | 180 ± 39 | 183 ± 56 | 0.635 |
| Triglycerides (mg/dl) | 130 ± 67 | 127 ± 63 | 125 ± 65 | 0.928 |
| Low-density lipoprotein cholesterol (mg/dl) | 109 ± 41 | 115 ± 29 | 122 ± 50 | 0.466 |
| High-density lipoprotein cholesterol (mg/dl) | 43.6 ± 13.1 | 40.3 ± 12.1 | 38.1 ± 14.4 | 0.316 |
The angiographic findings are summarized in Table 2 . No significant differences were found in the infarct-related arteries, lesion location, or lesion length. However, multivessel disease was most common in the lowest CrCl group. The minimal lumen diameter was the smallest and the diameter stenosis was the greatest in the lowest CrCl group. No significant differences were found in the stent type used or the stent diameter; however, the stent length was longest and the number of deployed stent was the greatest in the lowest CrCl group. A trend was seen toward post-stenting no-reflow observed most frequently in the lowest CrCl group (8.5% [13 of 153] vs 11.7% [12 of 103] vs 20.4% [11 of 53]; p = 0.064).
| Variable | CrCl (ml/min) Group | p Value | ||
|---|---|---|---|---|
| ≥70 (n = 153) | 30–69 (n = 103) | <30 (n = 53) | ||
| Infarct-related coronary artery | 0.237 | |||
| Left main | 1 (0.7%) | 3 (2.9%) | 1 (1.9%) | |
| Left anterior descending | 81 (52.9%) | 54 (52.4%) | 32 (59.3%) | |
| Left circumflex | 18 (11.8%) | 13 (12.6%) | 11 (20.4%) | |
| Right | 53 (34.6%) | 33 (32.0%) | 10 (18.5%) | |
| Coronary lesion location | 0.829 | |||
| Ostium | 3 (2.0%) | 1 (1.0%) | 0 (0.0%) | |
| Proximal | 57 (37.3%) | 40 (38.8%) | 24 (44.4%) | |
| Middle | 80 (52.3%) | 50 (48.5%) | 25 (46.3%) | |
| Distal | 13 (8.5%) | 12 (11.7%) | 5 (9.3%) | |
| No. of diseased coronary arteries | 0.027 | |||
| 1 | 88 (57.5%) | 48 (46.6%) | 20 (37.0%) | |
| 2 | 38 (24.8%) | 25 (24.3%) | 23 (42.6%) | |
| 3 | 27 (17.6%) | 30 (29.1%) | 11 (20.4%) | |
| Multivessel coronary disease | 65 (42.5%) | 55 (53.4%) | 34 (63.0%) | 0.019 |
| Reference diameter (mm) | 3.09 ± 0.77 | 2.95 ± 0.72 | 2.86 ± 0.61 | 0.065 |
| Minimal lumen diameter (mm) | 0.94 ± 0.62 | 0.87 ± 0.45 | 0.76 ± 0.48 | 0.024 |
| Diameter stenosis (%) | 69.6 ± 16.7 | 70.5 ± 17.4 | 73.4 ± 20.3 | 0.028 |
| Lesion length (mm) | 18.0 ± 6.4 | 20.6 ± 7.5 | 22.0 ± 10.9 | 0.130 |
| Stent type | 0.269 | |||
| Sirolimus-eluting stent | 68 (44.4%) | 47 (45.6%) | 23 (42.6%) | |
| Paclitaxel-eluting stent | 21 (13.7%) | 22 (21.4%) | 6 (11.1%) | |
| Bare metal stent | 64 (41.8%) | 34 (33.0%) | 25 (46.3%) | |
| Stent diameter (mm) | 3.29 ± 0.46 | 3.23 ± 0.41 | 3.16 ± 0.46 | 0.148 |
| Stent length (mm) | 23.1 ± 9.8 | 25.1 ± 12.3 | 28.8 ± 11.2 | 0.024 |
| No. of deployed stents | 1.18 ± 0.45 | 1.15 ± 0.35 | 1.33 ± 0.55 | 0.032 |
The IVUS findings are summarized in Table 3 . The reference segment EEM and lumen CSAs were the smallest and the plaque plus media CSA and plaque burden were the greatest in the lowest CrCl group. The lesion site EEM and lumen CSAs were the smallest, the plaque plus media CSA and plaque burden were the greatest, and the IVUS lesion length was the longest in the lowest CrCl group. The arc of calcium was the greatest and the remodeling index was the smallest in the lowest CrCl group. Infarct-related artery plaque rupture and multiple plaque ruptures were the most common, the ruptured plaque cavities were the largest, and the plaque rupture length was the longest in the lowest CrCl group. IVUS-detected thrombus was observed most frequently in the lowest CrCl group.
| Variable | CrCl (ml/min) Group | p Value | ||
|---|---|---|---|---|
| ≥70 (n = 153) | 30–69 (n = 103) | <30 (n = 54) | ||
| Referent | ||||
| External elastic membrane cross-sectional area (mm 2 ) | 12.8 ± 4.5 | 12.2 ± 4.8 | 11.9 ± 4.5 | 0.034 |
| Lumen cross-sectional area (mm 2 ) | 8.5 ± 3.0 | 7.7 ± 3.0 | 7.0 ± 3.1 | 0.008 |
| Plaque plus media cross-sectional area (mm 2 ) | 4.3 ± 2.5 | 4.5 ± 2.8 | 4.9 ± 2.2 | 0.041 |
| Plaque burden (%) | 30.1 ± 12.3 | 36.9 ± 11.4 | 41.2 ± 10.8 | 0.003 |
| Lesion site | ||||
| External elastic membrane cross-sectional area (mm 2 ) | 12.7 ± 4.3 | 12.4 ± 5.1 | 11.1 ± 4.4 | 0.047 |
| Lumen cross-sectional area (mm 2 ) | 2.6 ± 1.2 | 2.5 ± 1.5 | 2.0 ± 1.1 | 0.017 |
| Plaque plus media cross-sectional area (mm 2 ) | 10.0 ± 4.0 | 9.9 ± 4.7 | 9.1 ± 4.1 | 0.024 |
| Plaque burden (%) | 77.4 ± 11.0 | 79.8 ± 12.5 | 82.0 ± 10.3 | 0.031 |
| Lesion length (mm) | 20.9 ± 9.1 | 23.1 ± 9.5 | 26.3 ± 9.6 | 0.038 |
| Plaque morphology | 0.065 | |||
| Soft | 82 (53.6%) | 44 (42.7%) | 20 (37.0%) | |
| Fibrotic | 26 (17.0%) | 16 (15.5%) | 10 (18.5%) | |
| Calcific | 31 (20.3%) | 34 (33.0%) | 19 (35.2%) | |
| Mixed | 14 (9.2%) | 9 (8.7%) | 5 (9.3%) | |
| Arc of calcium (°) | 103 ± 96 | 142 ± 110 | 180 ± 114 | <0.001 |
| Remodeling index | 0.99 ± 0.23 | 1.02 ± 0.22 | 0.93 ± 0.19 | 0.031 |
| Plaque rupture | 48 (31.4%) | 35 (34.0%) | 29 (53.7%) | 0.011 |
| Multiple plaque rupture | 17 (11.1%) | 13 (12.6%) | 18 (33.3%) | <0.001 |
| Plaque cavity area (mm 2 ) | 1.98 ± 0.89 | 2.20 ± 1.45 | 3.06 ± 1.70 | 0.002 |
| Ruptured plaque length (mm) | 2.33 ± 0.93 | 2.59 ± 1.50 | 3.33 ± 1.76 | 0.008 |
| Intravascular ultrasound-detected thrombus | 35 (22.9%) | 24 (23.3%) | 22 (40.7%) | 0.027 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


