Treatment of left main coronary artery bifurcation lesions might depend on the ostial left circumflex (LC) or ostial left anterior descending (LAD) disease severity. We sought to evaluate whether intravascular ultrasound assessment of the side branch ostium requires direct imaging or is accurate from the main vessel. Our retrospective analysis included 126 patients with left main coronary artery bifurcation disease (plaque burden ≥40% by intravascular ultrasound scanning). We analyzed pullbacks from the LAD and the LC. First, during the main vessel pullback (ie, from the LAD), we evaluated the side branch ostium (ie, of the LC). Second, we compared this oblique view with the direct ostial measurements during LC pullback. Finally, we repeated this process, imaging the ostial LAD from the LC. From the LAD, the oblique LC ostial lumen diameter was 3.0 ± 0.8 mm compared to the directly measured lumen diameter of 2.9 ± 0.6 mm. From the LC, the oblique LAD ostial lumen diameter was 2.9 ± 1.1 mm compared to the directly measured lumen diameter of 2.8 ± 0.5 mm. However, Bland-Altman plots showed significant variation in the oblique versus direct comparisons. The 95% limits of agreement ranged from −1.84 to 1.14 mm (mean difference −0.35, SD 0.75) for the LAD and −1.69 to 1.22 mm (mean difference −0.23, SD 0.73) for the LC. The “oblique view” detection of any plaque in the side branch predicted 40% or 70% plaque burden with good sensitivity but poor specificity. In conclusion, intravascular ultrasound evaluation of a side branch ostium from the main vessel is only moderately reliable, especially for distal left main coronary artery lesions. For an accurate assessment of the side branch ostium, direct imaging is necessary.
Percutaneous coronary intervention (PCI) to treat left main coronary artery (LMCA) lesions might be equivalent to coronary artery bypass graft surgery, especially in the drug-eluting stent era—irrespective of lesion location. The MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty versus Surgical Revascularization) registry enrolled 1,102 patients who underwent stent implantation and 1,138 patients who underwent bypass surgery. In the propensity score-matched cohort, no significant difference was found between stenting and bypass surgery in the risk of death or the composite end point of death, Q-wave myocardial infarction, or stroke—even though >50% of stent-treated patients had distal LMCA bifurcation lesions. These findings were supported by the prespecified LMCA subset analysis from SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery Trial). Although coronary angiography has been the reference standard for the evaluation of coronary artery disease, including selecting PCI versus surgery and selecting among different PCI strategies, it has many limitations compared to intravascular ultrasound (IVUS) evaluations. Intravascular ultrasound also has limitations, including artifacts, transducer angulation or eccentric location, and anterograde-retrograde (systole-diastole) transducer movement that must be considered when interpreting images and making measurements. One concern during LMCA PCI is disease of the side branch ostium; however, interventionalists have been reluctant to perform IVUS scans of both daughter vessels, relying instead on an oblique view of the side branch from the main vessel. We considered whether IVUS imaging of the main vessel (typically from the left anterior descending [LAD] to the LMCA) could reliably assess the lumen dimensions or even the disease extent at the ostium of the side branch (typically the left circumflex [LC]).
Methods
The study consisted of an inclusive group of 126 patients who underwent diagnostic angiography and preinterventional IVUS assessment of the LMCA bifurcation, including imaging of the LAD and LC arteries. The patients were selected if the angiographic findings suggested compromise of the LMCA bifurcation and if IVUS imaging showed a plaque burden of ≥40% in any of the 3 components of the bifurcation (distal LMCA, ostial LAD, or ostial LC). The cohort included 45 patients with an acute coronary syndrome (not attributed to the LMCA) who were enrolled in the multicenter, prospective, international Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial ( ClinicalTrials.gov identifier no. NCT00180466 ) and who fit the above criteria. All patients provided written informed consent. The remaining patients came from Columbia University Medical Center (New York, New York; n = 48) and Showa University Northern Yokohama Hospital (Yokohama, Japan; n = 34). Patient demographics were confirmed by hospital chart review at the time of the procedure. The coronary risk factors included diabetes mellitus (controlled by diet, oral medication, or insulin), hypertension (medication-treated only), hyperlipidemia (medication-treated or a measurement of >220 mg/dl), current cigarette smoking, and renal insufficiency (defined as serum creatinine ≥1.3 mg/dl).
All angiograms (before the introduction of a guidewire into either daughter vessel) were reviewed by 2 experienced observers without knowledge of the clinical or IVUS data. All segments <10 mm from the distal LMCA bifurcation were analyzed with a computer-assisted, automated edge-detection algorithm (CMS, Medis, Leiden, The Netherlands). End-diastolic frames after intracoronary nitroglycerin administration were chosen for quantitative analysis. The average diameter of the proximal and distal angiographically normal segments was used as the reference diameter. The minimum lumen diameter and lesion length were calculated as the average of 2 orthogonal views. An ostial lesion was defined as a narrowing located <3 mm of the vessel origin on the least-foreshortened angiographic projection. Among multiple angiographic projections, the worst angle between the LAD and LC was measured using a protractor.
All studies were performed before PCI after the administration of 200 μg of intracoronary nitroglycerin using 1 of 2 commercially available IVUS catheters (40-MHz catheter, Boston Scientific, Natick, Massachusetts, and 20-MHz catheter, Eagle Eye, Volcano, Rancho Cordova, California) and corresponding imaging systems. IVUS imaging included the parent vessel (LMCA) and both daughter vessels (LAD and LC). In every patient, imaging of the daughter vessels began in the distal artery with motorized pullback (0.5 mm/s) through the distal LMCA bifurcation to the aorto-ostial junction. The IVUS images were recorded onto 0.5-in., high-resolution s-VHS videotape or digital media for off-line analysis.
The analyzed segments included the distal 10 mm of the LMCA and the proximal 10 mm of the LAD and LC. In each pullback (ie., from the LAD to the LMCA), we measured the ostium of the primary daughter vessel (ie, the LAD) directly and the ostium of the side branch (ie, the LC) by (1) measuring its lumen diameter using a line corresponding to the smallest lumen diameter after visual inspection and (2) assessing qualitatively the presence of significant plaque burden (yes/no; Figure 1 ). We then repeated this imaging process from the LC to the LMCA, with the LC as the primary daughter vessel and the LAD as the side branch. We then compared the oblique ostial measurements with the direct ostial measurements that included both the minimum lumen diameter and the mean direct lumen diameter calculated from the square root of the lumen area. Finally, we determined whether the presence or absence of plaque in the side branch (on the oblique view from the main vessel) predicted a plaque burden of 40% and 70% when the daughter vessel was imaged directly.
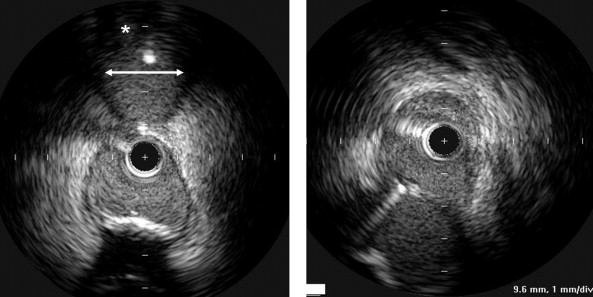
Qualitative and quantitative analyses were done according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular Ultrasound Studies. Using planimetry software (EchoPlaque, INDEC Systems, Mountain View, California), the IVUS measurements of the lumen, external elastic membrane, and plaque and media (external elastic membrane minus the lumen) cross-sectional areas and plaque burden (plaque and media divided by the external elastic membrane) were performed for each ostium (LAD, LC) and every 1 mm in the selected segments. The eccentricity index was the maximum divided by the minimum plaque and media thickness.
Statistical analysis was performed using StatView, version 5.0 (SAS Institute, Cary, North Carolina). The data are shown as the median and twenty-fifth/seventy-fifth quartiles. Regression analysis and Bland-Altman assessment for agreement were used to compare the 2 measurement methods. A range of agreement is shown as the mean difference ± 2 SDs. The Bland-Altman plot showed the agreement or disagreement between 2 different measurement methods for the same variable, usually the reference standard versus a new one, using a plot of the difference between the methods against their mean. In the present analysis, the Bland-Altman plot showed how much the oblique side branch ostium assessment was likely to differ from the direct assessment. If this difference was not enough to cause problems in clinical interpretation and decision making, we could replace the direct view by the oblique view or use the 2 interchangeably.
Results
The clinical patient characteristics are listed in Table 1 . The results of the quantitative coronary analysis are listed in Table 2 . The minimal diameter stenosis ranged from 1.2% to 69.8% for the distal LMCA, 4.2% to 82.7% for the first 10 mm of the proximal LAD segment, and 4.7% to 67.8% for the first 10 mm of the proximal LC segment.
| Variable | n (%) |
|---|---|
| Men | 55 (67%) |
| Age (years) | 67 ± 10 |
| Hypertension | 57 (70%) |
| Hyperlipidemia | 50 (61%) |
| Diabetes mellitus | 29 (35%) |
| Current smoker | 28 (61%) |
| Previous coronary artery bypass grafting | 6 (7.4%) |
| Variable | LMCA | LAD | LC |
|---|---|---|---|
| Reference vessel diameter (mm) | 4.3 (3.8–5.2) | 3.2 (2.9–3.9) | 3.2 (2.7–3.9) |
| Minimum lumen diameter (mm) | 3.2 (2.6–4.1) | 2.2 (1.7–2.7) | 2.1 (1.6–2.7) |
| Diameter stenosis (%) | 22 (15–36) | 33 (25–44) | 34 (27–45) |
The direct measurements for each ostium are listed in Table 3 . On IVUS assessment, 201 ostial lesions were found with a directly measured plaque burden of ≥40% (115 in the LAD and 86 in the LC), of which 24 ostial LAD lesions and 23 ostial LC lesions had a directly measured lumen area of ≤4 mm 2 .
| Variable | LAD | LC | ||||
|---|---|---|---|---|---|---|
| Ostium | Minimum Lumen Site | Reference | Ostium | Minimum Lumen Site | Reference | |
| Lumen area (mm 2 ) | 6.2 (5.1–7.9) | 5.2 (3.8–6.5) | 8.2 (6.4–10.6) | 6.3 (4.7–8.7) | 4.3 (3.1–6.4) | 7.9 (5.7–10.3) |
| External elastic membrane area (mm 2 ) | 14.4 (12.2–17.0) | 13.4 (11.7–16.3) | 14.6 (12.3–17.4) | 12.4 (10.1–14.7) | 10.5 (8.2–13.9) | 12.2 (9.3–15.6) |
| Plaque burden (%) | 54 (46–64) | 64 (54–70) | 44 (37–50) | 47 (36–57) | 55 (46–65) | 33 (27–41) |
| Minimum lumen diameter (mm) | 2.5 (2.1–2.8) | 2.2 (1.9–2.6) | 2.9 (2.5–3.2) | 2.5 (2.1–3.0) | 2.1 (1.7–2.6) | 2.9 (2.5–3.4) |
| Eccentricity index | 11.4 (7.4–19.1) | 9.0 (4.7–15.9) | NA | 6.8 (4.7–9.8) | 7.7 (4.7–11.5) | NA |
The evaluation of the side branch ostium from the main vessel pullback was possible in all but 1 patient. From the LAD, the oblique LC ostial lumen diameter was 3.0 ± 0.8 mm compared to the direct-measured diameter of 2.9 ± 0.6 mm. From the LC, the oblique LAD ostial lumen diameter was 2.9 ± 1.1 mm compared to the direct-measured diameter of 2.8 ± 0.5 mm. However, Bland-Altman analysis indicated that the 95% limits of agreement between the 2 methods ranged from −1.84 to 1.14 mm (mean difference −0.35, SD 0.75) for the LAD oblique versus the direct evaluation and from −1.69 to 1.22 mm (mean difference −0.23, SD 0.73) for the LC oblique versus the direct evaluation ( Figure 2 ). Although these 2 methods (direct vs oblique) provided similar average measurements, we considered the range of differences to be clinically important; therefore, we interpreted these data as showing a lack of agreement between the direct versus oblique view such that the oblique view should not be used in place of the direct view (or the 2 should not be used interchangeably). Furthermore, Figure 2 also shows the poor correlation between the direct and oblique views.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


