Up to 1/3 of percutaneous coronary interventions (PCIs) are complicated by troponin release. Remote ischemic preconditioning (IPC) confers effective cardioprotection; however, a 30-minute remote IPC protocol may be difficult to implement during ad hoc PCI. This study was performed to assess the ability of a brief remote IPC protocol to attenuate cardiac troponin I (cTnI) release after ad hoc PCI. Ninety-four patients undergoing ad hoc PCI for stable coronary artery disease, with undetectable preprocedural cTnI, were recruited and randomized to receive remote IPC (induced by one 5-minute inflation of a blood pressure cuff to 200 mm Hg around the upper arm) or control after the decision for PCI was made. The primary outcome was the difference between cTnI levels 24 hours after PCI and cTnI levels before coronary angiography (ΔcTnI). ΔcTnI in the remote IPC group was significantly lower compared with the control group (0.04 ng/ml [interquartile range 0.01 to 0.14] vs 0.19 ng/ml [interquartile range 0.18 to 0.59], p <0.001). The incidence of PCI-related myocardial infarction (MI) was greater in the control group (42.6% vs 19.1%, p = 0.014). In multivariate analysis, remote IPC was independently associated with ΔcTnI and PCI-related MI. In conclusion, our results suggest that even 1 cycle of remote IPC immediately before ad hoc PCI attenuates periprocedural cTnI release and reduces the incidence of type 4a MI.
Over 1/3 of percutaneous coronary interventions (PCIs) are complicated by periprocedural myocardial injury, detected by cardiac troponin I (cTnI) release, which is associated with subsequent cardiovascular events. Remote ischemic preconditioning (IPC) with 3 cycles of 5-minute ischemia and 5-minute reperfusion has been shown to effectively reduce myocardial injury in the setting of elective PCI. In the setting of ad hoc PCI, which is currently performed in most patients, the introduction of a 30-minute interval between the diagnostic angiogram and the PCI is not feasible. Because evidence from animal studies suggests that IPC is a graded and not an all-or-nothing phenomenon and that even preconditioning with 1 brief ischemic interval is as effective as preconditioning with multiple ischemic periods, we have conducted a single-center, randomized clinical trial to test the hypothesis that remote IPC with one 5-minute cycle of ischemia and subsequent reperfusion reduces myonecrosis during ad hoc PCI for stable coronary artery disease.
Methods
Consecutive patients admitted for coronary angiography for stable coronary artery disease, from July 2011 to May 2012, were identified and assessed for eligibility. All patients aged ≥18 years with a significant stenosis in coronary angiography, who were undergoing ad hoc PCI, and were able to give informed consent were eligible for the study. Exclusion criteria were (1) emergency PCI, (2) cTnI >0.04 ng/ml at the time of admission, (3) use of nicorandil or glibenclamide, and (4) renal dysfunction, defined as glomerular filtration rate ≤60 ml/min/1.73 m 2 .
All patients had to provide a signed informed consent to be included in the study. The study was approved by the local ethics committee and was registered to the ClinicalTrials.gov database ( NCT01158716 ). All participants had a blood pressure cuff placed around their nondominant arm. When the decision for PCI was made, patient allocation was revealed and those patients randomized to remote IPC had the cuff inflated to a pressure of 200 mm Hg for 5 minutes, followed by 1 minute of reperfusion before guiding catheter advancement. Control patients had a similar cuff placed around their arm, but it was not inflated.
All patients received acetylsalicylic acid 300 mg and clopidogrel 300 mg at least 6 hours (or 600 mg of clopidogrel >2 hours) before PCI. Unfractionated heparin was administered as an intravenous bolus at a dose of 70 to 100 IU/kg after arterial sheath insertion to achieve an activated clotting time of >250 seconds. Use of glycoprotein IIb/IIIa inhibitors was at the operators’ discretion. All patients received drug-eluting stents. After the procedure, all patients received acetylsalicylic acid 100 mg and clopidogrel 75 mg. All other medication was given at the discretion of the attending physician, and the PCI strategy was at the discretion of the interventional cardiologist according to conventional practice.
During PCI, chest pain severity was assessed with a 10-point scale (0: no pain, 10: most severe discomfort ever experienced) and the electrocardiogram was monitored for ST-segment deviation.
Venous blood samples were obtained before coronary angiography and at 24 hours after PCI. cTnI was analyzed with an automated immunoassay (Bayer ADVIA IMS Troponin-I Ultra method; Bayer, Berlin, Germany). The ninety-ninth percentile of the cTnI level in a reference population (upper reference limit) of healthy volunteers was below the lower limit of detection of 0.04 ng/ml. The coefficient of variation of the assay was <10%, complying with recent recommendations for optimal precision. The analytical range was 0.01 to 50 ng/ml, with an assay sensitivity of 0.006 ng/ml. According to the current definition of a PCI-related myocardial infarction (MI; MI type 4a), a cutoff of >0.20 ng/ml was predetermined (5 times the upper reference limit). Serum creatinine at the time of admission was used to estimate the glomerular filtration rate with the Modification of Diet in Renal Disease formula.
The volume of myocardium subtended by a stenosis, that is, the myocardium at risk, was assessed by reference to the target vessel using the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) lesion score.
Angiographic lesion characteristics were classified according to the modified American Heart Association/American College of Cardiology classification. The final result of stent implantation was assessed by quantitative coronary angiography. Postprocedural assessment of Thrombolysis In Myocardial Infarction flow score was performed by 2 interventional cardiologists, blinded to the cTnI results. Other periprocedural factors, such as length and type of implanted stent, duration and pressure of balloon inflations, or complications (coronary artery dissection, perforation, or jailed side branch with compromised flow), were recorded.
The primary outcome measure was ΔcTnI, defined as cTnI at 24 hours after PCI minus cTnI before coronary angiography. Secondary outcomes were the effect of remote IPC on ischemic symptoms and electrocardiographic evidence of ischemia during coronary balloon occlusion.
The sample size was determined assuming periprocedural ΔcTnI in the remote IPC group equal to 0.06 ng/ml, according to available evidence during study design. A sample size of 74 patients would be needed to detect a 2 times higher periprocedural ΔcTnI in the control group (α = 0.05, β = 0.2, statistical power = 80%).
Patients were allocated to treatment groups according to a computer-generated randomization procedure. The allocations were kept in sealed envelopes. Once a decision for PCI was made for an eligible patient, the group allocation was released to the study coordinator.
Continuous variables are summarized as mean ± SD or median (interquartile range) and were compared using Student t test or Mann-Whitney U test, as appropriate. Categorical data are expressed as frequencies (percentages) and were compared using Pearson chi-square test or Fisher’s exact test. Spearman correlation coefficient (r s ) was used to assess variable relations. Using multiple linear regression and logistic regression, we investigated the association of ΔcTnI and type 4a MI with several parameters identified by univariate analysis. All analyses were performed with SPSS, version 21 (IBM Corporation, Armonk, New York).
Results
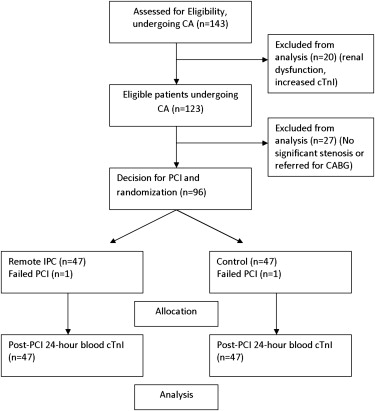
One hundred forty-three patients undergoing coronary angiography were assessed for eligibility. After excluding patients meeting prespecified criteria, a decision for ad hoc PCI was made for 96 patients, who were randomized into the 2 treatment groups ( Figure 1 ). Two patients (1 in each group) were excluded from the analysis because of PCI failure (failure to cross the lesion with the guidewire). Remote IPC was successfully administered to 47 patients without complications. Baseline clinical and demographic characteristics of patients randomized are reported in Table 1 .
| Variable | Control (n = 47) | Remote Ischemic Preconditioning (n = 47) | P |
|---|---|---|---|
| Demographics | |||
| Age (years) | 60.8 ± 10.4 | 60.2 ± 10.9 | 0.765 |
| Men | 41 (87%) | 42 (89%) | 0.748 |
| Risk factors | |||
| Diabetes mellitus | 8 (17%) | 10 (21%) | 0.600 |
| Dyslipidemia | 35 (75%) | 32 (68%) | 0.494 |
| Active or ex-smoker | 28 (60%) | 27 (58%) | 0.845 |
| BMI (kg/m 2 ) | 27.6 ± 2.8 | 29.1 ± 3.5 | 0.02 |
| Hypertension | 39 (83%) | 38 (81%) | 0.789 |
| Clinical details | |||
| LVEF (%) | 57.2 ± 9.9 | 55.5 ± 8.4 | 0.389 |
| Previous MI | 10 (21%) | 9 (19%) | 0.797 |
| CCS grade III/IV | 10 (21%) | 13 (28%) | 0.472 |
| GFR (mL/min/1.73 m 2 ) | 88.3 ± 17.9 | 88.4 ± 17.7 | 0.970 |
| Medications | |||
| β-blockers | 39 (83%) | 38 (81%) | 0.789 |
| ACEIs/ARBs | 33 (70%) | 35 (75%) | 0.645 |
| Statins | 46 (98%) | 44 (94%) | 0.617 |
There were no major procedure-related complications within the first 24 hours. Angiographic parameters were similar in both groups and are presented in Table 2 . The mean time between blood pressure cuff deflation and stent balloon inflation in the remote IPC group was 4.06 ± 0.55 minutes.
| Variable | Control (n = 47) | Remote Ischemic Preconditioning (n = 47) | P |
|---|---|---|---|
| Angiographic parameters | |||
| Target coronary artery | |||
| Left anterior descending | 23 (49%) | 20 (43%) | 0.612 |
| Circumflex | 6 (13%) | 5 (11%) | |
| Right | 6 (13%) | 11 (23%) | |
| Combined | 12 (26%) | 11 (23%) | |
| APPROACH score | 37.4 ± 18.3 | 32.0 ± 18.0 | 0.158 |
| Modified Rentrop score | 0.704 | ||
| 0 | 36 (77%) | 39 (83%) | |
| 1 | 9 (19%) | 7 (15%) | |
| 2/3 | 2 (4%) | 1 (2%) | |
| Lesion type (AHA/ACC) | 0.492 | ||
| A | 14 (30%) | 13 (28%) | |
| B | 23 (49%) | 19 (40%) | |
| C | 10 (21%) | 15 (32%) | |
| Stenosis severity, % | 85.8 ± 8.6 | 83.6 ± 8.1 | 0.203 |
| Side branch >2 mm | 16 (34%) | 20 (43%) | 0.396 |
| Total Stent Length (mm) | 23 (15-28) | 26 (12-32) | 0.713 |
| Systolic blood pressure (mm Hg) | 132 ± 18 | 129 ± 14 | 0.371 |
| Diastolic blood pressure (mm Hg) | 76 ± 9 | 73 ± 9 | 0.174 |
| Heart rate (bpm) | 68.9 ± 9.9 | 71.2 ± 8.6 | 0.237 |
| Chest pain score >1 | 28 (60%) | 22 (47%) | 0.301 |
| ECG ST deviation >1 mm | 18 (38%) | 13 (28%) | 0.380 |
| Complications | |||
| Dissection | 2 (4%) | 1 (2%) | 1.00 |
| Jailed side branch | 4 (9%) | 3 (6%) | 0.677 |
| Post-procedural coronary flow | |||
| TIMI flow score | |||
| 0-2 | 7 (15%) | 1 (2%) | 0.059 |
| 3 | 40 (85%) | 46 (98%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


