The aim of the present study was to evaluate the factors that modulate the protective action of statins on cardiorenal function, regardless of the lipid-lowering effect. To treat abnormal serum lipid profiles, low-dose pitavastatin (1.0 mg/day) was administered to 65 hyperlipidemic patients. The exclusion criteria included left ventricular ejection fraction <40% and apparent renal disease. Age- and gender-matched patients with hyperlipidemia (n = 40) served as the controls. After 12 to 16 weeks of pitavastatin treatment, pitavastatin had decreased low-density lipoprotein cholesterol (from 143.5 ± 31.4 to 98.2 ± 19.4 mg/dl, p <0.01), triglycerides (from 157.7 ± 57.2 to 140.5 ± 60.7 mg/dl, p <0.01), E/e′ (from 10.8 ± 6.2 to 9.0 ± 4.5, p <0.05), a parameter of left ventricular diastolic function, and albuminuria (from 47.6 ± 55.9 to 28.5 ± 40.0 mg/g creatinine, p <0.01). Furthermore, pitavastatin decreased serum transforming growth factor-β1 (from 709 ± 242 to 550 ± 299 pg/ml, p <0.01), urinary 8-hydroxy-2′-deoxyguanosine (from 6.6 ± 4.1 to 5.0 ± 3.1 μg/g creatinine, p <0.01), an oxidative stress marker, and increased urinary nitrate and nitrite (from 22.5 ± 14.6 to 29.4 ± 27.6 nmol/g creatinine, p <0.05). No such changes were observed in the controls. Multiple regression analysis in the pitavastatin group revealed the effect of pitavastatin on cardiorenal function was associated with suppression of oxidative stress, but not on low-density lipoprotein cholesterol reduction. In conclusion, pitavastatin decreases E/e′ and albuminuria, which is associated with suppression of oxidative stress.
Cardiovascular and renal diseases are closely related to each other as a cardiorenal syndrome; however, a strategy for the management of this syndrome has not yet been established. We previously reported that pitavastatin exerts endothelial nitric oxide synthase-independent protective actions against angiotensin II-induced cardiorenal syndrome through suppression of oxidative stress. Pitavastatin is lipophilic and is hardly metabolized by the liver, suggesting that pitavastatin directly interacts with cardiovascular systems and exerts cardiorenal protective effects even at a low dose. In the present study, we tested the hypothesis that low-dose pitavastatin has cardiorenal protective effects in hypercholesterolemic patients without severe cardiorenal dysfunction.
Methods
We enrolled 65 patients with hypercholesterolemia whose serum lipid profiles did not reach the management goals promulgated by the 2007 guidelines for the prevention of atherosclerotic cardiovascular diseases of the Japanese Atherosclerosis Society. In primary prevention, patients are categorized into low-, intermediate-, and high-risk groups, depending on the number of cardiovascular risk factors, other than low-density lipoprotein (LDL) cholesterol, and the LDL cholesterol goal was <160, <140, and <120 mg/dl, respectively. In secondary prevention, the LDL cholesterol goal was <100 mg/dl. The goal of high-density lipoprotein (HDL) cholesterol and triglycerides is ≥40 mg/dl and <150 mg/dl, regardless of the number of cardiovascular risk factors, respectively. The controls were 40 age- and gender-matched patients with hypercholesterolemia, with 1:1 or 1:2 matching. They chose diet and exercise therapy during the study period after receiving sufficient explanation about the study protocol. The study subjects were outpatients and hospitalized patients of the Department of Cardiovascular Medicine of Tokushima University hospital. The 2 groups did not significantly differ with respect to the patient characteristics or parameters of cardiac echocardiography ( Tables 1 and 2 ). The exclusion criteria included severe left ventricular (LV) dysfunction (LV ejection fraction <40%), atrial fibrillation, apparent renal disease (serum creatinine >2.0 mg/dl, urinary albumin excretion >300 mg/g creatinine), clinical conditions that could lead to increased inflammatory cytokine levels (i.e., rheumatoid arthritis), and liver disease, as defined by hepatic enzymes >2 times the upper normal limit. Also excluded were patients who were taking lipid-lowing agents, patients who were newly medicated, and patients whose medications for hypertension and dyslipidemia had been changed <1 year before the start of the present study. All subjects provided informed consent before enrollment in the study in accordance with protocols approved by the Tokushima University Hospital Ethics Committee. The pitavastatin group received low-dose pitavastatin (1.0 mg/day) orally and continued exercise and/or diet therapy. The control group also continued exercise and/or diet therapy after enrollment in the present study. During the study period, the patients’ medication regimens were unchanged, except for receiving pitavastatin. Before and 12 to 16 weeks after the start of the study, echocardiography was performed to assess LV systolic function, as determined by the LV ejection fraction, and to assess LV diastolic function, according to the ratio of peak transmitral flow velocities of E/A and the ratio of peak E velocity to early diastolic mitral annulus velocity (E/e′) as a preload independent index of LV filling pressure ( Table 2 ). Also, blood and urine samples were collected after an overnight fast. The lipid parameters, including LDL cholesterol, triglycerides, HDL cholesterol, and total cholesterol (TC) were assayed using enzymatic methods, and the non-HDL cholesterol and TC/HDL cholesterol ratio were calculated. The estimated glomerular filtration rate and urinary albumin, urinary excretion levels of nitrate and nitrite (as a parameter of nitric oxide bioavailability), 8-hydroxy-2′-deoxyguanosine (as a parameter of oxidative stress), and high-sensitivity C-reactive protein (hs-CRP) were assessed as previously described. Serum transforming growth factor-β1 was measured using an enzyme-linked immunosorbent assay (Human TGF-β1, R&D Systems, Minneapolis, Minnesota).
| Variable | Control (n = 40) | Pitavastatin (n = 65) |
|---|---|---|
| Age (years) | 64.7 ± 11.4 | 66.2 ± 12.1 |
| Men | 20 (50.0%) | 33 (50.8%) |
| Hypertension | 29 (72.5%) | 47 (72.3%) |
| Diabetes mellitus/impaired glucose tolerance | 15 (37.5%) | 29 (44.6%) |
| Coronary artery disease ⁎ | 19 (47.5%) | 34 (52.3%) |
| Current smoker | 6 (15.0%) | 4 (6.2%) |
| Body mass index (kg/m 2 ) | 23.1 ± 3.2 | 23.7 ± 5.7 |
| Systolic blood pressure (mm Hg) | 137.3 ± 18.5 | 134.1 ± 17.6 |
| Diastolic blood pressure (mm Hg) | 77.8 ± 11.9 | 77.8 ± 11.4 |
| Mean blood pressure (mm Hg) | 98.2 ± 12.2 | 96.6 ± 11.9 |
| Heart rate (beats/min) | 67.9 ± 7.6 | 68.1 ± 6.8 |
| Total cholesterol (mg/dl) | 228.7 ± 30.0 | 218.0 ± 36.9 |
| Low-density lipoprotein cholesterol (mg/dl) | 145.5 ± 27.3 | 143.5 ± 31.4 |
| Triglyceride (mg/dl) | 161.5 ± 64.8 | 157.7 ± 57.2 |
| High-density lipoprotein cholesterol (mg/dl) | 55.6 ± 12.3 | 50.8 ± 11.3 |
| Nonhigh-density lipoprotein cholesterol (mg/dl) | 173.1 ± 26.3 | 167.2 ± 38.4 |
| Total cholesterol/high-density lipoprotein cholesterol ratio | 4.3 ± 0.8 | 4.5 ± 1.3 |
| Fasting plasma glucose (mg/dl) | 119.0 ± 16.1 | 111.1 ± 17.1 |
| Hemoglobin A1c (%) | 5.9 ± 0.8 | 6.0 ± 0.9 |
| Estimated glomerular filtration rate (mL/min/1.73 m 2 ) | 71.8 ± 40.4 | 70.8 ± 41.0 |
| Urinary excretion of albumin (mg/g creatinine) | 40.1 ± 48.9 | 47.6 ± 55.9 |
| High-sensitivity C-reactive protein (μg/dl) | 206 ± 198 | 235 ± 253 |
| Urinary excretion of nitrate and nitrite (nmol/g creatinine) | 25.6 ± 18.3 | 22.5 ± 14.6 |
| Urinary excretion of 8-hydroxy-2′-deoxyguanosine (μg/g creatinine) | 6.2 ± 4.3 | 6.6 ± 4.1 |
| Transforming growth factor-β1 (pg/ml) | 699 ± 240 | 709 ± 242 |
| Current medication | ||
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 25 (62.5%) | 42 (64.6%) |
| Calcium channel blockers | 16 (40.0%) | 24 (36.9%) |
| β Blockers | 3 (7.5%) | 10 (15.4%) |
| Aldosterone antagonists | 1 (2.5%) | 2 (3.1%) |
⁎ Coronary artery disease defined as angina pectoris, myocardial infarction, and silent myocardial ischemia with or without percutaneous coronary intervention or coronary artery bypass surgery.
| Variable | Control (n = 40) | Pitavastatin (n = 65) |
|---|---|---|
| Left atrial diameter (cm) | 3.7 ± 0.7 | 3.8 ± 0.7 |
| Left ventricular end-diastolic volume (ml) | 83 ± 23 | 86 ± 26 |
| Left ventricular end-systolic volume (ml) | 30 ± 15 | 31 ± 15 |
| Left ventricular ejection fraction (%) | 65 ± 8 | 65 ± 7 |
| E (cm/s) | 59 ± 18 | 61 ± 20 |
| A (cm/s) | 73 ± 23 | 76 ± 19 |
| E/A | 0.88 ± 0.45 | 0.83 ± 0.28 |
| e′ (cm/s) | 6.7 ± 2.3 | 6.6 ± 2.6 |
| E/e′ | 10.0 ± 4.1 | 10.8 ± 6.2 |
Natural log transformation of urinary albumin excretion, hs-CRP, and 8-hydroxy-2′-deoxyguanosine was used for statistical analysis, because the data were not normally distributed. The paired t test and unpaired t test were used for within-group and between-group comparisons of the lipid profiles and other biomarkers, respectively. All data are expressed as the mean ± SD. Multiple regression analysis was used to assess the correlations between a decrease in E/e′ or albuminuria and the other indexes in the control and pitavastatin groups. These analyses were performed using a Microsoft Windows computer running Statistical Package for Social Sciences software (SPSS, Chicago, Illinois). Differences were considered statistically significant at p <0.05.
Results
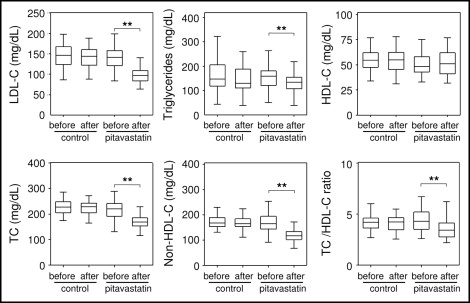
Pitavastatin significantly reduced LDL cholesterol (percentage of change −31.6%, p <0.01), triglyceride (percentage of change −10.9%, p <0.01), TC (percentage of change −21.5%, p <0.01), non-HDL cholesterol (percentage of change −28.7%, p <0.01), and TC/HDL cholesterol ratio (percentage of change −23.2%, p <0.01) but had no effect on HDL cholesterol. In the controls, we observed no significant differences in the measurements obtained before and after the start of the study ( Figure 1 ). Significant differences were found in the temporal changes between the groups in LDL cholesterol, triglycerides, TC, non-HDL cholesterol, and TC/HDL cholesterol ratio but not in HDL cholesterol.
Pitavastatin treatment significantly decreased E/e′, a sensitive and preload-independent parameter of LV diastolic function (percentage of change −16.7%, p <0.05), but not E/A. Pitavastatin did not affect LV systolic function as indicated by the LV ejection fraction ( Figure 2 ) and blood pressure. The controls showed no differences in the measurements obtained before and after the start of the study ( Figure 2 ). No significant differences were seen in the temporal changes between the groups in LV ejection fraction, E/A, or E/e′.
Pitavastatin treatment attenuated urinary albumin excretion (percentage of change −40.1%, p <0.01) without changing the estimated glomerular filtration rate. The controls showed no changes after the start of the study ( Figure 3 ). Significant differences were seen in the temporal changes between the groups in urinary albumin excretion but not in the estimated glomerular filtration rate.




