It remains unknown whether left ventricular (LV) reverse remodeling (LVRR) after therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and β blockers is correlated with prognosis in patients with idiopathic dilated cardiomyopathy. Forty-two patients with idiopathic dilated cardiomyopathy treated with the therapy were studied. Complete left ventricular reverse remodeling was defined as LV end-diastolic dimension ≤55 mm and fractional shortening ≥25% at the last echocardiographic assessment. The incidence of complete LVRR was significantly higher in patients who survived than in those who died or underwent heart transplantation. Patients were divided into 3 groups: death or transplantation, alive with complete LVRR, and alive without complete LVRR. Although patients who died or underwent transplantation did not show any LV improvements, those with complete LVRR showed significant improvements at 1 to 6 months after starting the therapy. Patients without complete LVRR also showed small but significant improvements at 1 to 6 months. The decrease in LV end-systolic dimension from the initial value to that at 1 to 6 months was an independent determinant of future cardiac death or transplantation. In conclusion, complete LVRR is related to favorable prognosis in patients with idiopathic dilated cardiomyopathy. The extent of left ventricular reverse remodeling at 1 to 6 months after starting the therapy is predictive of long-term prognosis.
The prognosis of patients with idiopathic dilated cardiomyopathy (IDC) is not as bad as before the use of β blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs). However, it is important to identify patients who still have poor outcomes. Although there are many prognostic predictors, they are not sensitive in individual patients. Left ventricular (LV) remodeling has an important role in disease progression and prognosis of IDC. Beta blockers, in addition to ACE inhibition, reduce LV volume with an improvement in systolic function, a phenomenon known as LV reverse remodeling (LVRR). The appearance of LVRR is generally thought to result in a favorable prognosis. Nonetheless, it is unclear whether there is an association between the extent of LVRR and prognosis. The aim of the present study was to determine whether LVRR after therapy with ACE inhibitors or ARBs and β blockers is correlated with long-term prognosis in patients with IDC.
Methods
We retrospectively studied 42 patients with IDC who were treated with therapy with ACE inhibitors or ARBs and β blockers. All patients were admitted to our hospital for confirmation of diagnosis, risk assessment, and symptom management during the period from 1994 to 2005. Our study was approved by the Ethics Committee on Medical Research of Kochi Medical School. An exhaustive clinical evaluation, including medical history, physical examination, 12-lead electrocardiography, ambulatory 24-hour electrocardiography, laboratory studies, echocardiography, and cardiac catheterization, was performed in each patient to identify the cause of cardiomyopathy as precisely as possible. The diagnostic criteria were (1) dilated LV end-diastolic dimension (LVDd) >55 mm with LV fractional shortening (LVFS) <25% and (2) exclusion of patients with acute myocarditis, infiltrative myocardial disease, connective tissue disease, endocrine dysfunction, neuromuscular disease, general systemic disease, significant coronary artery stenosis (defined as diameter narrowing >50% in any of the major coronary arteries or their branches), valvular disease, sensitivity or toxic reactions, and histories of excessive alcohol intake. The estimated glomerular filtration rate was calculated. Nonsustained ventricular tachycardia was defined as ≥3 consecutive premature complexes at a mean rate of >120 beats/min. Echocardiography was performed before starting therapy with ACE inhibitors or ARBs and β blockers. LVDd, LV end-systolic dimension (LVDs), thicknesses of the interventricular septum and LV posterior wall, and left atrial dimension were measured by M-mode echocardiography as recommended by the American Society of Echocardiography. LVFS was calculated as [(LVDd − LVDs)/LVDd] × 100.
After admission, ACE inhibitors or ARBs were first initiated in addition to conventional pharmacologic therapy. Afterward, β blockers were administered. Either carvedilol or metoprolol tartrate was used in this study, because metoprolol succinate has not been available in Japan. The initial dose of carvedilol was 1.25 or 2.5 mg/day and that of metoprolol was 5 or 10 mg/day. Carvedilol or metoprolol was titrated to a targeting dose depending on the tolerability of each patient. The targeting dose of carvedilol was 20 mg/day and that of metoprolol was 80 mg/day. Dose titration was deferred or stepped back because of worsening symptoms, hypotension (systolic blood pressure <90 mm Hg), or bradycardia (heart rate at rest <50 beats/min). Echocardiography was repeated at least twice after starting the therapy except for 1 patient who died within 1 year. The definition of a marked reversal of LV remodeling in a previous study was referred and modified in the present study. We defined complete LVRR, which is normalization of LV systolic function, as LVDd ≤55 mm and LVFS ≥25% at the last echocardiographic assessment. Follow-up data were obtained by regular visits at our hospital, interviews with referring physicians and chart reviews, and telephone contact with the patients or their relatives. End points were cardiac death or heart transplantation. The study closed on December 31, 2006.
Categorical variables are presented as total number and percentage of patients, and continuous variables are presented as means ± SD. Fisher’s exact test was used to analyze the incidence of complete LVRR. Differences in initial variables among the 3 groups were checked with 1-way analysis of variance or the Kruskal-Wallis test or using contingency table analysis. Changes in LVDd, LVDs, and LVFS were evaluated using Wilcoxon’s signed-rank test. Differences in ΔLVDd, ΔLVDs, and ΔLVFS among the 3 groups were also analyzed using analysis of variance or the Kruskal-Wallis test. Scheffé’s F procedure or the Steel procedure was used as a post hoc test. The relations of cardiac death or transplantation with initial echocardiographic variables (LVDd, LVDs, and LVFS) and ΔLVDs were analyzed using multivariate Cox proportional-hazards model and are expressed as hazard ratios and 95% confidence intervals. Statistical significance was defined by a p value <0.05.
Results
The patients were aged 23 to 78 years (mean 59 ± 12), and 37 of the patients (88%) were men. Thirty-two patients were in New York Heart Association functional class I or II, and 10 patients were in class III or IV. Initial echocardiography showed that the mean LVDd was 65 ± 7 mm, the mean LVDs was 56 ± 8 mm, and the mean LVFS was 14 ± 5%. Clinical outcomes and the incidence of complete LVRR during a mean follow-up period of 4.7 ± 2.9 years (range 5 months to 11 years) are shown in Figure 1 . Eight patients died (heart failure death in 5 patients and sudden cardiac death in 3 patients), 1 underwent heart transplantation, and 33 survived. Hospitalization because of deterioration of congestive heart failure occurred in 1 patient who survived. Although 14 of the 33 patients (42%) who survived showed complete LVRR, none of those who died or underwent transplantation showed complete LVRR (p <0.05). In patients who showed complete LVRR, changes in LVDd, LVDs, and LVFS from the initial values to those at the last follow-up were as follows: for LVDd, 62 ± 6 to 49 ± 4 mm (p <0.005); for LVDs, 53 ± 6 to 33 ± 4 mm (p <0.005); and for LVFS, 15 ± 4% to 32% ± 4% (p <0.01). The decrease in LVDd was 14 ± 5 mm (range 6 to 24), and that in LVDs was 20 ± 6 mm (range 11 to 32). The increase in LVFS was 17 ± 7% (range 6% to 27%).
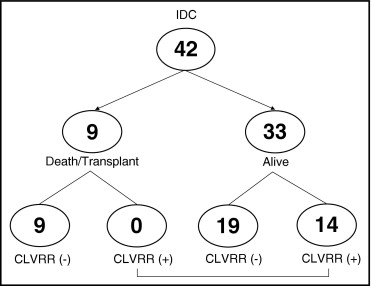
We divided the patients into 3 groups: (1) death or transplantation, (2) alive without complete LVRR, and (3) alive with complete LVRR. Clinical characteristics and medical treatments in the 3 groups are listed in Table 1 . There were no differences in these variables at initial evaluation. There were no significant differences in the frequency of use of ACE inhibitors or ARBs. We most frequently used enalapril (92%) as an ACE inhibitor and losartan (67%) as an ARB. The mean maintenance doses were 4.9 ± 1.3 mg/day (range 2.5 to 10) for enalapril and 43.8 ± 12.5 mg/day (range 25 to 50) for losartan. There were no significant differences in these maintenance doses among the 3 groups. Carvedilol was administered in 36 patients and metoprolol in 6 patients. There were no significant differences in the frequency of use of these drugs. The mean maintenance doses were 10.1 ± 5.0 mg/day (range 2.5 to 20) for carvedilol and 64.0 ± 21.9 mg/day (range 40 to 80) for metoprolol. There were no significant differences in these maintenance doses among the 3 groups. The mean duration between the initiation of ACE inhibitors or ARBs and that of β blockers was 7.5 ± 1.1 days (range 5 to 10). There were no differences in the duration among the 3 groups. AACE inhibitors or ARBs and β blockers were continued during follow-up in all patients, although there were some changes of the other concomitant drugs, such as diuretics, when clinically indicated. However, these changes were not statistically different among the 3 groups. Initial echocardiographic and cardiac catheterization findings are listed in Table 2 . There were no significant differences in these findings.
| Death or Transplantation | Alive Without Complete LVRR | Alive With Complete LVRR | |
|---|---|---|---|
| Variable | (n = 9) | (n = 19) | (n = 14) |
| Age (years) | 58 ± 14 | 58 ± 12 | 60 ± 11 |
| Men/women | 8/1 | 17/2 | 12/2 |
| New York Heart Association class | |||
| I or II | 7 | 16 | 9 |
| III or IV | 2 | 3 | 5 |
| Atrial fibrillation | 1 (11%) | 3 (16%) | 5 (36%) |
| Nonsustained ventricular tachycardia | 3 (33%) | 8 (42%) | 5 (36%) |
| Serum creatinine (mg/dl) | 0.80 ± 0.27 | 0.81 ± 0.26 | 0.85 ± 0.16 |
| Estimated glomerular filtration rate (ml · min −1.73 m −2 | 82.8 ± 23.9 | 82.9 ± 29.3 | 71.7 ± 14.6 |
| Serum hemoglobin (g/dl) | 14.0 ± 1.3 | 14.6 ± 1.1 | 14.6 ± 1.3 |
| Follow-up period (years) | 4.3 ± 3.9 | 4.4 ± 2.6 | 5.2 ± 2.5 |
| Medical treatment | |||
| β blockers | 9 (100%) | 19 (100%) | 14 (100%) |
| ACE inhibitors/ARBs | 8/1 (100%) | 16/3 (100%) | 12/2 (100%) |
| Loop diuretics | 9 (100%) | 18 (95%) | 12 (86%) |
| Spironolactone | 6 (67%) | 9 (47%) | 6 (43%) |
| Digitalis | 9 (100%) | 11 (57%) | 11 (78%) |
| Antiarrhythmic drugs | 4 (44%) | 5 (26%) | 4 (29%) |
| Variable | Death or Transplantation | Alive Without Complete LVRR | Alive With Complete LVRR |
|---|---|---|---|
| LVDd (mm) | 66 ± 6 | 67 ± 7 | 62 ± 6 |
| LVDs (mm) | 58 ± 7 | 57 ± 9 | 53 ± 6 |
| LVFS (%) | 13 ± 4 | 15 ± 5 | 15 ± 4 |
| Interventricular septal thickness (mm) | 10 ± 1 | 9 ± 2 | 10 ± 2 |
| LV posterior wall thickness (mm) | 9 ± 1 | 10 ± 2 | 10 ± 2 |
| Left atrial dimension (mm) | 44 ± 8 | 41 ± 7 | 43 ± 6 |
| LV end-diastolic volume index (ml/m 2 ) | 191 ± 46 | 161 ± 41 | 144 ± 72 |
| LV end-systolic volume index (ml/m 2 ) | 138 ± 38 | 113 ± 43 | 100 ± 66 |
| LV ejection fraction (%) | 28 ± 4 | 32 ± 9 | 34 ± 12 |
| LV end-diastolic pressure (mm Hg) | 17 ± 12 | 11 ± 5 | 12 ± 6 |
| Pulmonary capillary wedge pressure (mm Hg) | 16 ± 12 | 9 ± 4 | 12 ± 8 |
| Systolic pulmonary artery pressure (mm Hg) | 35 ± 11 | 27 ± 7 | 30 ± 11 |
| Mean pulmonary artery pressure (mm Hg) | 25 ± 14 | 17 ± 4 | 19 ± 8 |
| Right ventricular end-diastolic pressure (mm Hg) | 9 ± 6 | 7 ± 2 | 8 ± 3 |
| Mean right atrial pressure (mm Hg) | 7 ± 8 | 5 ± 2 | 6 ± 3 |
| Systolic aortic pressure (mm Hg) | 105 ± 15 | 115 ± 20 | 112 ± 21 |
| Mean aortic pressure (mm Hg) | 80 ± 11 | 86 ± 12 | 87 ± 15 |
| Cardiac index (ml/min/m 2 ) | 2.0 ± 0.5 | 2.3 ± 0.5 | 2.1 ± 0.5 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


