Several studies have demonstrated a relation between the rennin-angiotensin-aldosterone system and atrial fibrillation (AF), but there are no reports on the effect of eplerenone, a selective aldosterone blocker, on the prevention of AF recurrence after radiofrequency catheter ablation (RFCA). The aim of this study was to evaluate the effects of eplerenone on clinical outcomes after RFCA in patients with long-standing persistent AF. A total of 161 consecutive patients with long-standing persistent AF (sustained AF duration 1 to 20 years, mean 3.4 ± 3.8) who underwent RFCA were investigated. Eplerenone was used in 55 patients and not used in the remaining 106 patients. Other conventional pharmacologic agents, including angiotensin-converting enzyme inhibitors or angiotensin type 1 receptor blockers, were used equally in the 2 groups. After 24 months of follow-up, 47% of the patients were free from AF recurrence. The rate of freedom from AF recurrence was significantly greater in the eplerenone group (60%) than in the noneplerenone group (40%) (p = 0.011). By univariate analysis, the duration of sustained AF (p <0.001), left atrial diameter (p = 0.010), left atrial volume index (p = 0.017), and early AF recurrence (p <0.001) were significantly associated with AF recurrence, and the use of eplerenone was associated with maintenance of sinus rhythm after RFCA (p = 0.022). Multivariate Cox regression analysis showed that longer duration of sustained AF (>3 years) (p <0.001) and early AF recurrence (p <0.001) were significantly associated with AF recurrence, and only eplerenone therapy significantly improved maintenance of sinus rhythm (p = 0.017). In conclusion, eplerenone significantly improved maintenance of sinus rhythm after RFCA in patients with long-standing persistent AF.
Atrial fibrillation (AF), the most common sustained arrhythmia in clinical practice, is associated with increased risk for morbidity and mortality. Radiofrequency catheter ablation (RFCA) has been established as effective therapy for drug-refractory AF, but a significantly high recurrence rate remains a problem to be resolved in long-standing persistent AF. The rennin-angiotensin-aldosterone (RAA) system, which has been shown to promote inflammation and fibrosis, is one of the major factors in the progression of arrhythmogenic atrial remodeling. Suppression of the RAA system has been proved to prevent AF susceptibility by attenuating atrial structural remodeling. However, recent randomized clinical studies failed to show a benefit of angiotensin type 1 receptor blockers (ARBs) for the prevention of AF recurrence. It was assumed that angiotensin-converting enzyme (ACE) inhibitors or ARBs alone cannot suppress the production of aldosterone completely. Therefore, we hypothesized that eplerenone, a selective aldosterone blocker, might have favorable effects in the prevention of AF recurrence after RFCA. The aim of this study was to evaluate the effect of treatment with eplerenone on maintenance of sinus rhythm (SR) after RFCA in patients with long-standing persistent AF.
Methods
This retrospective observational study was conducted to investigate the efficacy of eplerenone in preventing AF recurrence after RFCA. A total of 182 consecutive patients with long-standing persistent AF of >1 year in duration who had no histories of AF ablation and underwent RFCA at the University of Tsukuba Hospital (Tsukuba, Japan) from January 2008 to July 2010 were included. Among these patients, we excluded those meeting the following criteria: (1) had moderate to severe valvular heart disease and left ventricular systolic dysfunction (left ventricular ejection fraction <40%) (n = 4), (2) could not be followed up (n = 6), (3) lacked complete data (n = 3), and (4) discontinued use of eplerenone (n = 8). Thus, a total of 161 patients were included in the analysis. The mean duration of sustained AF was 3.4 ± 3.8 years (range 1 to 20), and all patients had histories of unsuccessful treatment with an average of 3.8 ± 1.9 antiarrhythmic drugs (AADs).
Before ablation, all patients underwent careful clinical evaluation that included a detailed medical history including medications and physical examination. Duration of sustained AF was defined as a period of sustained AF obtained by precise examination of medical history, including several 24-hour Holter recordings and serial 12-lead electrocardiograms recorded at every patient visit. Hypertension was defined as (1) systolic blood pressure ≥140 mmHg, (2) diastolic blood pressure ≥90 mmHg, or (3) receiving antihypertensive medication for hypertension. Dyslipidemia was defined as (1) a fasting blood plasma low-density lipoprotein level ≥140 mg/dl, fasting blood plasma high-density lipoprotein level <40 mg/dl, or fasting blood plasma triglyceride level ≥150 mg/dl or (2) receiving lipid-lowering medication for dyslipidemia. Drinking habit was defined as drinking ≥22 g of pure alcohol per day and ≥3 days a week, in accordance with the National Health and Nutrition Examination Survey conducted by the Ministry of Health, Labor and Welfare of Japan.
Plasma concentrations of high-sensitivity C-reactive protein, B-type natriuretic peptide, and serum creatinine were also evaluated according to our previous study. The estimated glomerular filtration rate was calculated as previously described. Standard echocardiography was performed using a Vivid 7 system (GE Vingmed Ultrasound AS, Horten, Norway). Left atrial (LA) volume was measured with a planimeter from the apical 4- and 2-chamber views at ventricular end-systole by tracing the LA endocardial border (biplane modified Simpson’s method), excluding the pulmonary veins and LA appendage.
All AADs were discontinued ≥5 half-lives before ablation, except for amiodarone, which was discontinued ≥6 weeks beforehand. All patients had received warfarin as anticoagulation therapy with a target international normalized ratio of 2.0 to 3.0. All patients underwent transesophageal echocardiography (iE33; Phillips Medical Systems, Andover, Massachusetts) to rule out the presence of thrombus before the ablation procedure. Intravenous heparin was administered to maintain an activated clotting time of 350 to 400 seconds. RFCA with extensive circumferential pulmonary vein isolation was performed by a double-lasso technique, as previously described. After a transseptal catheterization, 2 7Fr 10-polar ring catheters (Lasso; Biosense Webster, Inc., Diamond Bar, California) and a 7.5Fr open-irrigation catheter with a 3.5-mm distal electrode (ThermoCool; Biosense Webster, Inc.) were positioned in the left atrium. The 2 ring catheters were positioned at each pulmonary vein ostium for mapping of pulmonary vein potentials. Radiofrequency energy was delivered using the temperature-control mode with a target temperature of 42°C and maximum power output of 25 W for the posterior left atrium and 35 W for the anterior aspects. The end point of RFCA was the creation of an extensive ipsilateral bidirectional conduction block from the atrium to the pulmonary veins and vice versa and confirmation of bidirectional conduction block ≥60 minutes after successful pulmonary vein isolation. If AF was sustained after pulmonary vein isolation, additional ablation, consisting of a linear ablation of the LA roof, superior vena cava isolation, and/or ablation of complex fractionated atrial electrogram ablation, was performed. If AF did not terminate after that additional ablation, SR was restored by internal cardioversion. A cavotricuspid isthmus ablation line was also created in all patients, with confirmation of bidirectional conduction block.
Patients remained hospitalized under continuous rhythm monitoring for about 4 days after the procedure and were then followed at 2 weeks after ablation and then every 1 to 2 months at our outpatient clinic, as previously reported. At each hospital visit, patients underwent 12-lead electrocardiography and intensive questioning regarding any arrhythmia-related symptoms. Twenty-four-hour Holter monitoring and portable electrocardiographic monitoring (HCG-901R; Omron, Kyoto, Japan) for 3 minutes twice daily on 3 consecutive days were also performed at 1, 3, 6, 14, and 24 months after RFCA. Subsequently, a 12-lead electrocardiogram and a 24-hour Holter electrocardiogram were also recorded whenever patients reported palpitations. If the electrocardiograms showed any episodes of AF during follow-up, patients were diagnosed as having clinical recurrences of AF; however, AF recurrence within the first 3 months after RFCA was defined as early AF recurrence and was only considered transient, and a blanking period of 3 months was applied. Patients with transient AF recurrence within these first 3 months were treated only temporarily with class I, II, III, and/or IV AADs, and discontinuation of the AADs was then attempted in all patients in whom the recurrent AF disappeared after temporary treatment. Repeat ablation during the blanking period was defined as AF recurrence. The end point of this study was defined as recurrent AF or atrial tachycardia. Ethical approval for this study was obtained from the local review committee, and all patients provided written informed consent.
Continuous variables are expressed as mean ± SD. Comparisons between 2 groups were tested using unpaired Student’s t test. All categorical variables were tested using chi-square analysis or Fisher’s exact tests to detect differences between groups. Comparison of the probability of freedom from AF recurrence between patients with and without eplerenone was performed using Kaplan-Meier survival analysis with the log-rank test. Cox proportional-hazards analysis was applied to explore any predictors of AF recurrence. Multivariate analysis was performed with variables that were statistically significant in the univariate analysis and previously reported predictors of AF recurrence. A p value <0.05 was considered significant. All statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, Illinois).
Results
RFCA was successfully performed in all patients. During the 24-month follow-up period, 74 patients (86%) had AF recurrence and 12 patients had atrial tachycardia occurrence. During the first 3 months after RFCA, 82 patients had AF or atrial tachycardia recurrence. After transient AAD therapy and/or external cardioversion, 18% of these patients converted to and maintained SR. In the other 82% of these patients, AF or atrial tachycardia continued or recurred during the 24-month follow-up period ( Figure 1 ).
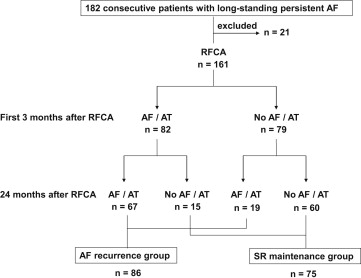
A comparison of the baseline characteristics between AF recurrence and SR maintenance groups is shown in Table 1 . AF duration was significantly longer and LA diameter and LA volume index were significantly larger in the AF recurrence group than in the SR maintenance group ( Table 1 ). The patients with early AF recurrence within the 3-month blanking period were more frequently liable to AF recurrence than SR maintenance ( Table 1 ). However, no significant differences were found between the 2 groups in terms of the other variables ( Table 1 ). Additional ablation procedures, consisting of linear ablation of the LA roof, superior vena cava isolation, and complex fractionated atrial electrogram ablation, were performed equally between the 2 groups, with no major complications ( Table 2 ).
| Variable | Total (n = 161) | SR Maintenance (n = 75) | AF Recurrence (n = 86) | p Value |
|---|---|---|---|---|
| Age (yrs) | 60.5 ± 9.6 | 59.5 ± 8.4 | 61.4 ± 8.7 | 0.17 |
| Men | 134 (83%) | 59 (79%) | 75 (87%) | 0.15 |
| Body mass index (kg/m 2 ) | 25.2 ± 3.3 | 25.3 ± 3.7 | 25.2 ± 2.9 | 0.98 |
| Hypertension | 81 (50%) | 40 (53%) | 41 (48%) | 0.47 |
| Dyslipidemia | 89 (55%) | 44 (59%) | 45 (52%) | 0.42 |
| Diabetes mellitus | 30 (19%) | 18 (24%) | 12 (14%) | 0.10 |
| Smoking | 93 (58%) | 45 (60%) | 48 (55%) | 0.59 |
| Drinking habit | 88 (55%) | 40 (53%) | 48 (55%) | 0.75 |
| Structural heart disease | 19 (12%) | 10 (13%) | 9 (10%) | 0.57 |
| Ischemic heart disease | 9 (6%) | 4 (5%) | 5 (6%) | 0.59 |
| Dilated cardiomyopathy | 5 (3%) | 2 (3%) | 3 (4%) | 0.57 |
| Hypertrophic cardiomyopathy | 3 (2%) | 2 (3%) | 1 (1%) | 0.45 |
| Valvular heart disease | 2 (1%) | 2 (3%) | 0 (0%) | 0.22 |
| Duration of sustained AF (yrs) | 3.4 ± 3.8 | 2.3 ± 2.1 | 4.4 ± 4.6 | <0.001 ∗ |
| Systolic blood pressure (mmHg) | 123.8 ± 14.4 | 123.9 ± 15.0 | 123.8 ± 13.9 | 0.96 |
| Diastolic blood pressure (mmHg) | 71.9 ± 10.6 | 70.4 ± 10.7 | 73.2 ± 10.4 | 0.10 |
| Plasma B-type natriuretic peptide (pg/ml) | 95.3 ± 89.9 | 90.8 ± 94.8 | 99.2 ± 85.7 | 0.56 |
| Serum high-sensitivity C-reactive protein (mg/dl) | 0.18 ± 0.40 | 0.16 ± 0.47 | 0.19 ± 0.32 | 0.69 |
| Serum potassium (mEq/L) | 4.2 ± 0.33 | 4.2 ± 0.35 | 4.3 ± 0.30 | 0.16 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 71.4 ± 15.6 | 73.5 ± 16.9 | 69.4 ± 14.1 | 0.11 |
| Echocardiographic parameters | ||||
| Left ventricular ejection fraction (%) | 64.0 ± 9.2 | 64.8 ± 8.6 | 63.3 ± 9.6 | 0.28 |
| LA diameter (mm) | 41.8 ± 6.7 | 40.1 ± 7.1 | 43.3 ± 5.9 | 0.002 ∗ |
| LA volume index (ml/m 2 ) | 38.8 ± 14.4 | 35.5 ± 13.4 | 41.7 ± 14.7 | 0.006 ∗ |
| Interventricular septal thickness (mm) | 9.1 ± 1.5 | 9.1 ± 1.3 | 9.2 ± 1.7 | 0.84 |
| Left ventricular posterior wall thickness (mm) | 9.2 ± 1.4 | 9.0 ± 1.3 | 9.3 ± 1.5 | 0.29 |
| Early AF recurrence | 82 (51%) | 15 (20%) | 67 (78%) | <0.001 ∗ |
| Variable | Total (n = 161) | SR Maintenance (n = 75) | AF Recurrence (n = 86) | p Value |
|---|---|---|---|---|
| RFCA | ||||
| Additional catheter ablation procedure | ||||
| LA roof linear ablation | 99 (62%) | 47 (63%) | 52 (61%) | 0.78 |
| Complex fractionated atrial electrogram ablation | 71 (44%) | 33 (44%) | 38 (44%) | 0.98 |
| Superior vena cava isolation | 36 (22%) | 18 (24%) | 18 (21%) | 0.64 |
| Duration of radiofrequency energy applications (min) | 58.5 ± 17.5 | 56.5 ± 16.8 | 60.4 ± 18.2 | 0.17 |
| Total radiofrequency energy delivered (kJ) | 90.7 ± 32.4 | 88.4 ± 27.5 | 92.7 ± 36.5 | 0.41 |
| Drug therapy | ||||
| Eplerenone | 55 (34%) | 33 (44%) | 22 (26%) | 0.014 ∗ |
| β blockers | 107 (67%) | 46 (61%) | 61 (71%) | 0.20 |
| ACE inhibitors/ARBs | 93 (58%) | 41 (55%) | 52 (61%) | 0.46 |
| Calcium channel blockers | 34 (21%) | 20 (27%) | 14 (16%) | 0.11 |
| Statins | 75 (47%) | 36 (48%) | 39 (45%) | 0.74 |
| Diuretics | 12 (8%) | 5 (7%) | 7 (8%) | 0.72 |
The patients without AF recurrence were more frequently treated with eplerenone than were those with AF recurrence ( Table 2 ). In this study, eplerenone was administered to 26 of 81 patients with hypertension. However, 29 of the 55 patients in the eplerenone group used eplerenone as an aspect of upstream therapy at the discretion of each physician. Among 55 patients taking eplerenone, we found no adverse effects to be associated with eplerenone in this study. There was no significant difference in other medications, including ACE inhibitors or ARBs, β blockers, calcium channel blockers, statins, and diuretics between the 2 groups ( Table 2 ).
The overall rate of freedom from AF recurrence at 24 months after RFCA was 47%. We further investigated the midterm success rate of the patients with or without eplerenone. Kaplan-Meier curves of freedom from AF recurrence in the eplerenone and noneplerenone groups are shown in Figure 2 . The rate of SR maintenance remained significantly higher in the eplerenone group (60%) than in the noneplerenone group (40%) at the end of 24 months of follow-up ( Figure 2 ).
Factors associated with AF recurrence were investigated by univariate analysis. Age (+10 years), baseline diastolic blood pressure (+10 mmHg), and the natural log of plasma B-type natriuretic peptide level were not associated with AF recurrence during the follow-up period. However, longer duration of sustained AF (>3 years), larger LA diameter (+1 mm), larger LA volume index (+1 ml/m 2 ), and early AF recurrence were significantly associated with AF recurrence, and only eplerenone therapy was significantly associated with SR maintenance ( Table 3 ). Furthermore, multivariate Cox regression analysis revealed that longer duration of sustained AF (>3 years) and early AF recurrence were significantly associated with AF recurrence. In addition, only eplerenone therapy was significantly associated with the reduction of AF recurrence during the 24-month follow-up period ( Table 3 ).
| Variable | Univariate Analysis | Multivariate Analysis ∗ | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (+10 yrs) | 1.200 (0.948–1.519) | 0.130 | 1.150 (0.915–1.445) | 0.232 |
| Duration of sustained AF (>3 yrs) | 2.196 (1.426–3.384) | <0.001 ∗ | 1.076 (1.029–1.125) | <0.001 ∗ |
| Diastolic blood pressure (+10 mmHg) | 1.189 (0.979–1.444) | 0.081 | 1.014 (0.993–1.036) | 0.204 |
| Natural log of plasma B-type natriuretic peptide level | 1.440 (0.838–2.473) | 0.179 | ||
| LA diameter (+1 mm) | 1.010 (1.010–1.074) | 0.010 ∗ | 1.021 (0.987–1.056) | 0.238 |
| LA volume index (+1 ml/m 2 ) | 1.017 (1.004–1.031) | 0.017 ∗ | ||
| Early AF recurrence | 5.994 (3.555–10.105) | <0.001 ∗ | 5.565 (3.269–9.475) | <0.001 ∗ |
| Eplerenone | 0.566 (0.348–0.920) | 0.022 ∗ | 0.537 (0.329–0.876) | 0.013 ∗ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


