Excessive atrial fibrosis is involved in the pathogenesis of atrial fibrillation (AF), but little is known of left ventricular (LV) fibrotic status in patients with AF. In the present study, we investigated the presence of abnormal LV fibrosis in AF, its effect on cardiac function, a possible association with arterial stiffness (i.e., systemic cardiovascular fibrosis), and the parameters of endothelial activation, dysfunction, and damage. We also studied whether LV fibrosis could be linked to the future risk of AF onset. In a cross-sectional study, the severity of LV fibrosis was assessed by echocardiographic acoustic densitometry in patients with permanent AF (n = 49), patients with paroxysmal AF (n = 44), AF-free “disease controls” (n = 42) and “healthy controls” (n = 48). Arterial stiffness (pulse wave velocity), plasma markers of endothelial activation (E-selectin), endothelial damage/dysfunction (von Willebrand factors), and microvascular endothelial function (laser Doppler flowmetry) were quantified. In a longitudinal study, 93 patients with pacemakers (22 with AF) were followed up for ≥1 year to assess the predictive value of LV fibrosis for the development of new-onset AF. More severe LV fibrosis was present in both paroxysmal and permanent AF than in the AF-free controls (p <0.001), with more LV fibrosis in permanent than in paroxysmal AF (p = 0.002). The severity of LV fibrosis in AF wais independently associated with diastolic dysfunction (p = 0.03), but not with LV contractility, arterial stiffness, or endothelial damage/dysfunction. In conclusion, LV fibrosis might contribute to LV diastolic dysfunction and the high prevalence of heart failure with preserved ejection fraction in subjects with AF.
In the present study, we investigated the presence of abnormal left ventricular (LV) fibrosis in atrial fibrillation (AF), its effect on cardiac function, its relation to arterial stiffness (i.e., systemic cardiovascular fibrosis), and indexes of endothelial activation, damage, and dysfunction. To address our objectives, we performed a cross-sectional study to compare the severity of LV fibrosis in patients with AF to that in AF-free subjects and to establish the associations with vascular abnormalities. We also performed a longitudinal study to assess the predictive value of LV fibrosis for AF development and the natural pattern of LV fibrosis progression in subjects with and without AF. Both studies were used to evaluate the association of LV fibrosis with LV systolic and diastolic function.
Methods
We studied patients with permanent AF (n = 49) and paroxysmal AF (n = 44). These patients were compared with AF-free “disease controls” (i.e., patients with a history of hypertension in sinus rhythm, n = 42) and “healthy controls” (n = 48; Table 1 ). The exclusion criteria included recent stroke, myocardial infarction, thromboembolism, uncontrolled hypertension, inflammatory or connective tissue disorders, active infection, malignancy, pregnancy, hepatic or renal impairment, and long-term immunosuppressive therapy.
| Variable | Permanent AF (n = 49) | Paroxysmal AF (n = 44) | Disease Controls (n = 42) | Healthy Controls (n = 48) | p Value |
|---|---|---|---|---|---|
| Age (yrs) | 69 ± 10 ∗,†,‡ | 59 ± 13 | 59 ± 10 | 55 ± 10 | <0.001 |
| Men | 33 (67%) | 28 (64%) | 27 (64%) | 34 (71%) | 0.88 |
| Systolic blood pressure (mm Hg) | 138 ± 18 ‡ | 137 ± 14 ‡ | 135 ± 16 ‡ | 126 ± 10 | <0.001 |
| Diastolic blood pressure (mm Hg) | 78 ± 12 | 77 ± 8 | 81 ± 10 | 77 ± 8 | 0.19 |
| Body mass index (kg/m 2 ) | 29 ± 5 | 28 ± 7 | 28 ± 4 | 29 ± 3 | 0.32 |
| Heart rate (beats/min) | 76 ± 11 ∗,†,‡ | 60 ± 9 | 63 ± 11 | 63 ± 10 | <0.001 |
| Hypertension | 39 (80%) | 40 (91%) | 39 (93%) | — | 0.11 |
| Diabetes mellitus | 6 (12%) | 9 (20%) | 10 (24%) | — | 0.39 |
| Hypercholesterolemia (>5 mmol/L) | 23 ± 47 | 29 ± 66 | 22 ± 52 | — | 0.17 |
| Coronary artery disease (confirmed by angiography) | 6 (12%) | 12 (27%) | 9 (21%) | — | 0.19 |
| Previous stroke | 6 (12%) | 7 (16%) | 7 (17%) | — | 0.81 |
| Smoker | 19 (39%) | 12 (27%) | 7 (17%) | — | 0.07 |
| Alcohol users | 30 (61%) | 19 (43%) | 23 (55%) | — | 0.21 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 27 (55%) | 30 (68%) | 20 (48%) | — | 0.15 |
| β Blockers | 23 (47%) | 19 (43%) | 10 (24%) | — | 0.10 |
| Aspirin | 19 (39%) | 21 (48%) | 18 (43%) | — | 0.66 |
| Warfarin | 31 (63%) | 16 (36%) | 1 (2%) | — | <0.001 |
| Diuretics | 7 (14%) | 11 (25%) | 16 (38%) | — | 0.03 |
| Statins | 20 (41%) | 26 (59%) | 25 (60%) | — | 0.12 |
∗ p <0.05 versus paroxysmal AF.
† p <0.05 versus disease controls.
For the longitudinal part of the study, we recruited 93 consecutive elderly subjects who were undergoing regular surveillance in our cardiology clinic >3 months after implantation of a cardiac pacemaker for sinus node or atrioventricular node disease. The exclusion criteria included previously documented permanent AF, pacemaker system dysfunction (e.g., persistent atrial under- or oversensing), LV ejection fraction (LVEF) <40%, significant valvular heart disease, and other significant chronic disorders. The study included 22 patients with AF and 71 AF-free subjects ( Table 2 ). All study subjects were followed up for 1 year (median 336 days, interquartile range 294-402) with repeated echocardiography and assessment of cardiac fibrosis. New cases of AF were recorded at the end of the follow-up period. The local research ethics committee approved both parts of our study, and all participants provided written informed consent.
| Variable | AF | p Value | |
|---|---|---|---|
| No (n = 71) | Yes (n = 22) | ||
| Age (yrs) | 71 ± 11 | 75 ± 10 | 0.16 |
| Men | 48 (68%) | 17 (77%) | 0.28 |
| Hypertension | 45 (63%) | 16 (73%) | 0.42 |
| Diabetes mellitus | 11 (15%) | 8 (36%) | 0.07 |
| Coronary artery disease | 30 (42%) | 7 (32%) | 0.38 |
| History of stroke/transient ischemic attack | 8 (11%) | 2 (9%) | 0.77 |
| New York Heart Association class | |||
| I | 29 (41%) | 8 (36%) | 0.43 |
| II | 20 (28%) | 10 (45%) | |
| III | 1 (1%) | 0 (0%) | |
| Hypercholesterolemia | 46 (75%) | 17 (77%) | 0.20 |
| Smoker | |||
| Current | 11 (15%) | 0 (0%) | 0.10 |
| Past | 27 (38%) | 8 (36%) | |
| Alcohol (>10 U/wk) | 27 (38%) | 8 (36%) | 0.89 |
| Body mass index (kg/m 2 ) | 26 ± 4 | 28 ± 5 | 0.26 |
| Heart rate (beats/min) | 72 ± 9 | 75 ± 10 | 0.24 |
| Systolic blood pressure (mm Hg) | 137 ± 23 | 140 ± 17 | 0.63 |
| Diastolic blood pressure (mm Hg) | 77 ± 11 | 77 ± 9 | 0.99 |
| Anigotensin-converting enzyme inhibitors or angiotensin receptor blockers | 39 (55%) | 16 (73%) | 0.16 |
| β Blockers | 22 (31%) | 7 (32%) | 0.97 |
| Aspirin | 46 (65%) | 14 (64%) | 0.86 |
| Clopidogrel | 9 (13%) | 0 | 0.07 |
| Warfarin | 6 (8%) | 6 (27%) | 0.02 |
| Diuretics | 23 (32%) | 8 (36%) | 0.75 |
| Statins | 42 (59%) | 16 (72%) | 0.45 |
Cardiac function was assessed by 2-dimensional echocardiography (Phillips iE33 ultrasound machine, Bothel, Washington). Modern off-line QLAB software (Xcelera, Phillips Ultrasound Quantification Module) was used for quantification of the parameters of cardiac structure and function. Diastolic function was assessed using the E/E′ ratio. The inter- and intra-assay variability for the measured echocardiographic parameters in our laboratory was <5%.
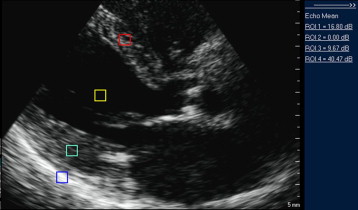
Measurement of integrated backscatter (IB) and calculation of calibrated IB (cIB) was performed using echocardiographic acoustic densitometry software (a part of the QLAB package). For the estimation of myocardial fibrosis, we used 2-dimensional images acquired from the parasternal long-axis view with frame rates of 80 to 120 frames/s. At least 3 cardiac cycles were stored in cine-loop format for off-line analysis. For measurement of LV cIB fixed-size (5 × 5-mm) regions of interest were positioned at the midmyocardium of the interventricular septum and the posterior wall and in the posterior pericardium at LV level as a control sample ( Figure 1 ). Measurements of the IB were performed by 2 operators who had not been involved in the scanning of the patients and were unaware of the patients’ status. LV cIB was calculated as an average or septal and posterior wall cIB values. The intra-assay coefficient of variability for LV cIB (measurements made on 5 subjects on 5 different days each) was 6.7%. The interassay coefficient of variability for LV cIB was 12.0% (on 25 subjects assessed by 2 operators who were unaware of the patients’ status).
To establish the possible association of LV fibrosis with arterial stiffness, we measured the carotid-femoral pulse wave velocity and aortic augmentation index using Sphygmocor device (Sphygmocor, Atcor Medical, Sydney, Australia). The carotid-femoral pulse wave velocity was obtained from sequential electrocardiographic-gated tonometer recordings at the carotid and femoral arteries. This technique was validated in our laboratory, and the inter- and intra-assay variability was 10% and 5.1%, respectively.
Assessment of microvascular endothelial function was performed using a scanning laser Doppler flowmeter (Periscan System PIM II, Perimed AB, Stockholm, Sweden; 780-nm red laser) with iontophoresis (0.1 mA for 60 seconds) of 2% acetylcholine (to evaluate the endothelial-dependent response) and 1% sodium nitroprusside (to evaluate the endothelium-independent response), as previously described in detail. This technique was validated in our laboratory, and the inter- and intra-assay variability (n = 10) was 6.3% and 8.0%, respectively.
E-selectin and von Willebrand factor were measured using commercial enzyme-linked immunosorbent assay according to the manufacturers’ recommendations (RD Systems, Abingdon, United Kingdom; and Dako, Ely, United Kingdom, respectively) using citrated plasma. The intra-assay and interassay coefficient of variation was <5% and <10%, respectively.
Data are expressed as the mean ± SD for normally distributed variables or median with first and third quartiles (interquartile range) for non-normally distributed variables. One-way analysis of variance with Tukey’s post hoc test was used for cross-sectional comparisons. t Tests were used for the longitudinal substudy. Appropriate nonparametric tests were used for non-normally distributed data. A p value (2-tailed) of <0.05 was considered statistically significant. Multivariate regression analysis has been performed to determine the factors associated with the parameters of cardiac fibrosis. SPSS, version 18 (SPSS, Chicago, Illinois) statistical software was used to perform the statistical analyses.
On the basis on our preliminary work on the study parameters (LV cIB), we calculated that a sample size of 35 subjects in each group for analysis of variance in the cross-sectional study and a minimum of 21 subjects for a t test would have 80% power to detect a difference of 0.5 SD in LV cIB.
Results
All study groups were matched by gender, but patients with permanent AF were significantly older than those from the other study groups ( Table 1 ). During the recruitment of consecutive patients with AF meeting the study criteria, an obvious age difference was seen between those with the paroxysmal and permanent forms of AF, reflecting the natural pattern of the disease. Accordingly, we choose to recruit controls to be age-matched with paroxysmal AF and to use multivariate analysis to explore whether any cardiac fibrotic changes in AF were independently associated with specific AF forms, after adjustment for age and other important variables.
All patients with AF and “disease controls” were matched for most clinical and demographic characteristics and treatments, except (as expected) a greater heart rate in those with permanent AF and greater warfarin usage in both AF groups (p <0.001; Table 1 ). Also, the study patients had higher systolic blood pressure than the healthy controls (p <0.001).
Patients with AF had an increased left atrial (LA) volume compared to the control groups (p <0.001). The LA volume was larger in those with permanent AF than in those with paroxysmal AF (p <0.001; Table 3 ). The LVEF was lower in subjects with permanent AF than in other groups (p <0.001). The E/E′ ratio was lower in the “healthy controls” than in the “disease controls” (p = 0.003) and patients with paroxysmal AF (p = 0.003) and permanent AF (p = 0.001).



