Restenosis is associated with acute myocardial infarction (MI) either at presentation or related to complications of target lesion revascularization (TLR). The cumulative late effect of TLR after drug-eluting or bare metal stent placement on cardiac death or MI is uncertain. Of the 1,057 patients with one native coronary lesion randomized to a sirolimus-eluting stent or bare metal stent in the Sirolimus-Eluting Stent in De Novo Native Coronary Lesions (SIRIUS) trial, the 983 who survived free of MI for the first 30 days were evaluated for the primary outcome of cardiac death or MI for 5 years. Patients with events occurring at or after TLR were assigned to TLR group. Cox proportional hazards regression analysis with TLR as a time-dependent variable and adjustment for baseline clinical and demographic covariates was used to assess the independent effect of TLR on the primary outcome. TLR occurred in 160 patients (16.3%) and was an independent predictor of the primary end point (hazard ratio 2.8, 95% confidence interval 1.7 to 4.5). This association was significant for sirolimus-eluting stents and bare metal stents. TLR was also associated with an increased risk of subsequent stent thrombosis and nontarget vessel revascularization. Intracoronary brachytherapy in the TLR group was associated with an increased risk of cardiac death or MI. In conclusion, restenosis requiring TLR was associated with an increased risk of cardiac death or MI occurring at TLR and during the subsequent 5 years.
Restenosis requiring repeat percutaneous or surgical revascularization procedures has been the major limitation of balloon angioplasty and bare metal stent (BMS) placement for coronary artery disease. Recent reports have indicated the frequent occurrence of myocardial infarction (MI) at repeat target lesion revascularization (TLR). Drug-eluting stents (DESs) have reduced the frequency of restenosis requiring TLR within the first year, ranging from 3% to 4% for DESs to 11% to 16% for BMSs in pivotal clinical trials. In 2006, reports were published of increased late mortality in patients receiving a DES. Subsequent patient-level pooled analyses of clinical trial data suggested a small increase in late stent thrombosis >1 year after stenting, but no increase in death or MI for DESs compared to BMSs. A post hoc analysis of pooled paclitaxel versus BMS studies reported equal numbers of deaths in patients who received either a BMS or a paclitaxel DES complicated by either TLR or stent thrombosis. The investigators hypothesized a balance between the small late mortality risk due to stent thrombosis and the accrued benefits of restenosis prevention in a larger number of patients. The direct late clinical effect of restenosis requiring TLR after stent implantation with DESs or BMSs has not been specifically investigated. The aim of the present study was to evaluate the effect of TLR performed for in-stent restenosis after BMS or sirolimus-eluting stent (SES) placement on long-term cardiac outcomes. We hypothesized that TLR would be associated with a significantly increased risk of subsequent cardiac events, defined as the composite end point of cardiac death or MI.
Methods
The study population included patients randomized in the Sirolimus-Eluting Stent in De Novo Native Coronary Lesions (SIRIUS) study. The design of the SIRIUS trial has been previously reported. After approval of the device by the United States Food and Drug Administration, the study patients were to be followed up for 5 years. The patients were queried by telephone interview, and, in the case of potential clinical events, the source medical documents were retrieved and reviewed. All study end points were adjudicated by an independent committee that remained masked to the treatment assignment.
The TLR group included patients who required clinically driven TLR for presumed restenosis at any point >30 days from the index procedure. The no-TLR group included patients who had remained free of clinically driven TLR throughout the follow-up period. Because TLR was treated as a time-dependent variable, the patients entered into the TLR group at TLR. The exception was that patients who experienced MI ≤7 days before TLR were considered to have MI related to restenosis and were entered into the TLR group at the time of the MI. TLR performed within the first 30 days from the index percutaneous coronary intervention was presumed to be secondary to a procedure-related complication rather than restenosis, and these patients remained in the no-TLR group unless subsequent TLR occurred after 30 days. TLR performed for stent thrombosis was likewise deemed not due to restenosis, and these patients also remained in the no-TLR group.
Clinically driven TLR was adjudicated if ischemic symptoms and a target lesion diameter stenosis >50% or a target lesion diameter stenosis >70% in the absence of ischemic symptoms were present. Diameter stenosis was determined using quantitative coronary angiography performed by a central core laboratory. The target lesion was defined as the coronary segment containing the stent plus a 5-mm proximal or distal margin.
The primary end point for the present analysis was a composite of cardiac death or any MI. Deaths were categorized as cardiac unless a clear noncardiac cause was identified. MI was defined according the Academic Research Consortium and Global Task Force recommendations. Periprocedural MI was defined as creatine kinase-MB >3 times the upper reference limit after percutaneous coronary intervention or creatine kinase-MB >5 times the upper reference limit after coronary artery bypass surgery. Spontaneous MI was defined as any signs or symptoms of acute myocardial ischemia with troponin or creatine kinase-MB greater than the upper reference limit.
The secondary end points included the individual components of the primary end point, nontarget vessel revascularization, and stent thrombosis (according to the Academic Research Consortium classification).
The data on continuous variables are presented as the mean ± SD if normally distributed and as the median and interquartile range if not normally distributed. The comparison of baseline characteristics between patients undergoing TLR and those who remained free of TLR was performed using the chi-square test or Fisher’s exact test for dichotomous outcomes and the Student t test for continuous outcomes. Event rates during the 5 years of follow-up were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazards regression analysis was used to evaluate the association of TLR with the primary end point, adjusted for baseline variables after confirmation of the proportional hazard assumptions. Considering TLR as a time-dependent group variable, patients contributed survival time to the no-TLR group until a TLR event occurred, at which point, they began contributing survival time to the TLR group.
All reported p values are 2-sided, and p <0.05 was considered significant.
Results
The patient population included eligible patients enrolled in the SIRIUS trial ( Figure 1 ). Of the 983 study-eligible patients, 160 (16.3%) underwent TLR for restenosis from day 31 through the 5 years of follow-up from the index procedure. The median interval to TLR was 246 days. The median follow-up duration in the TLR group after TLR was 1,550 days, and the median follow-up duration in no-TLR group was 1,801 days.
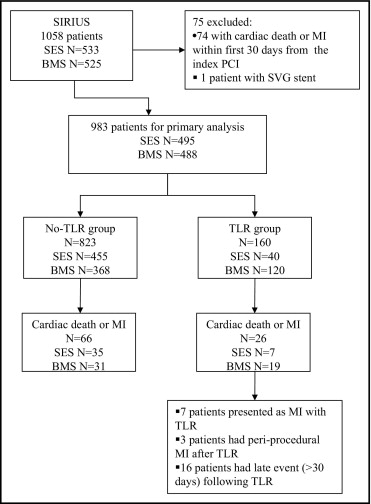
TLR was required in 8.1% of patients receiving a SES compared to 24.6% receiving a BMS (p <0.001). Patients with TLR had greater rates of diabetes and peripheral artery disease ( Table 1 ). In the TLR group, the treated lesions were longer, located in smaller diameter vessels, and more frequently located in the left anterior descending artery ( Table 2 ).
| Variable | TLR | p Value | |
|---|---|---|---|
| Yes (n = 160) | No (n = 823) | ||
| Age (years) | 61.3 ± 11.2 | 62.2 ± 11.1 | 0.33 |
| Men | 75.0% | 70.7% | 0.29 |
| Sirolimus-eluting stent | 25.0% | 55.3% | < 0.001 |
| Diabetes mellitus | 38.1% | 24.5% | < 0.001 |
| Hypertension (history or treatment) | 68.4% | 67.8% | 0.93 |
| Hyperlipidemia (history or treatment) | 76.6% | 73.3% | 0.43 |
| Current smoker | 19.4% | 20.4% | 0.68 |
| Renal dialysis | 0% | 0.6% | 1.00 |
| Stroke | 7.5% | 6.8% | 0.74 |
| Heart failure | 7.0% | 5.0% | 0.33 |
| Peripheral arterial disease | 12.5% | 6.4% | 0.01 |
| Previous myocardial infarction | 32.1% | 28.4% | 0.34 |
| Previous percutaneous coronary intervention | 26.3% | 24.2% | 0.62 |
| Previous bypass surgery | 12.5% | 8.9% | 0.18 |
| Ejection fraction (%) | 56.9 ± 10.2 | 55.7 ± 10.1 | 0.20 |
| No. of narrowed coronary arteries | 0.28 | ||
| 1 | 55.0% | 59.8% | |
| 2 | 28.8% | 26.0% | |
| 3 | 16.3% | 14.2% | |
| Variable | TLR | p Value | |
|---|---|---|---|
| Yes (n = 161) | No (n = 824) | ||
| Coronary lesion location | 0.01 | ||
| Left anterior descending artery | 53.8% | 41.0% | |
| Left circumflex | 23.8% | 24.9% | |
| Right | 22.5% | 34.1% | |
| American College of Cardiology-American Heart Association lesion classification | 0.16 | ||
| A | 6.9% | 7.4% | |
| B1 | 30.0% | 37.0% | |
| B2 | 37.5% | 32.9% | |
| C | 25.6% | 22.7% | |
| Coronary lesion characteristics | |||
| Diameter stenosis (%) | 64.8 ± 12.3 | 65.3 ± 12.3 | 0.67 |
| Total occlusion | 1.3% | 2.9% | 0.29 |
| Angiographic thrombus | 1.3% | 1.1% | 0.70 |
| Thrombolysis In Myocardial Infarction flow before procedure | 0.11 | ||
| 0 | 0.6% | 0.7% | |
| 1 | 0.6% | 2.2% | |
| 2 | 3.8% | 6.2% | |
| 3 | 95.0% | 90.9% | |
| Vessel diameter (mm) | 2.72 ± 0.45 | 2.82 ± 0.47 | 0.01 |
| Lesion length (mm) | 15.4 ± 7.3 | 14.1 ± 5.4 | 0.04 |
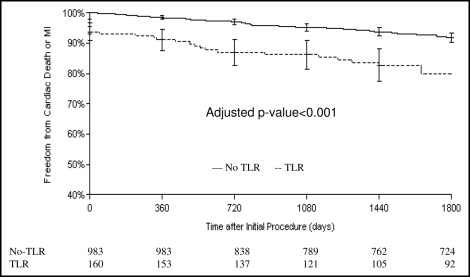
At 5 years, the cumulative incidence of cardiac death or MI was 16.6% in the TLR group and 8.4% in the patients who did not experience TLR (log-rank p = 0.0009; Figure 2 ). Patients who experienced cardiac death or MI were more likely to have a history of MI or diabetes and a smaller vessel diameter without other significant differences in the baseline clinical or lesion characteristics. The individual event hazard ratios (HRs) for the primary end point and other clinical outcomes are shown in Figure 3 . The association of TLR with cardiac death or MI was significant for both SES and BMS cohorts (p <0.001 for both). The attributable risk of cardiac death or MI related to TLR was similar for the SESs (7.1%) and BMSs (8.5%). The population attributable risk percentage (14.3% overall), however, was substantially greater for the BMS group (20.4% vs 6.7%), owing to a significantly greater proportion of patients undergoing TLR after BMS.
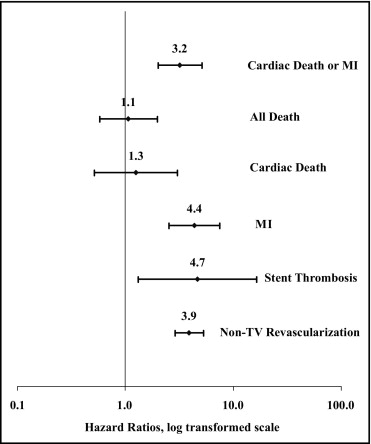
Multivariate adjustment using Cox proportional hazards regression analysis showed that the association of TLR with cardiac death or MI remained significant (HR 1.67, 95% confidence interval 1.67 to 4.52).
Of the 26 primary end point events in the TLR group, 16 occurred > 30 days after TLR. Table 3 lists the individual event descriptions of the 16 patients with late events. Most of the patients with a late event had undergone brachytherapy during the first or subsequent TLR (13 of 16, 81.3%) compared to 63 (47.1%) of 134 patients with TLR and no cardiac death or MI (p = 0.01). Of the 10 patients with a stent thrombosis event, 9 had received previous brachytherapy. Within the TLR group, the multivariate analysis for the prediction of late cardiac death or MI demonstrated that only brachytherapy performed at any point during the follow-up significantly increased the risk (HR 2.33, 95% confidence interval 1.01 to 5.36). Stent type was not significant (HR 0.55, 95% confidence interval 0.21 to 1.43).
| Pt. No. | TLR to Cardiac Death or MI (d) | Coronary Artery | Stent Type | Interval to TLR (d) | Brachytherapy | Cardiac Death or MI | Stent Thrombosis (ARC) |
|---|---|---|---|---|---|---|---|
| 1 | 47 | LAD | BMS | 231 | Yes | MI | Definite |
| 2 | 226 | Right | BMS | 251 | Yes | MI | Definite |
| 3 | 319 | Right | SES | 1,031 | No | MI | No |
| 4 | 331 | LC | SES | 210 | Yes | MI | No |
| 5 | 425 | Right | BMS | 651 | Yes | MI | No |
| 6 | 497 | LAD | SES | 122 | Yes | MI | Definite |
| 7 | 522 | LAD | BMS | 97 | Yes | MI | No |
| 8 | 548 | LAD | BMS | 251 | Yes | MI | Probable |
| 9 | 575 | LAD | BMS | 280 | No | CD | Possible |
| 10 | 637 | LC | BMS | 81 | Yes | CD | Possible |
| 11 | 869 | LAD | BMS | 143 | Yes | CD | Possible |
| 12 | 1,138 | LC | BMS | 248 | No | MI | No |
| 13 | 1232 | LAD | BMS | 250 | Yes | CD | Possible |
| 14 | 1304 | LC | BMS | 240 | Yes | MI | No |
| 15 | 1438 | LC | DES | 101 | Yes | CD | Possible |
| 16 | 1648 | LC | BMS | 72 | Yes | CD | Possible |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


