The aim of the present study was to analyze the effect of drug exposure patterns of clopidogrel and proton pump inhibitors (PPIs) on the clinical outcomes after percutaneous coronary intervention (PCI). Previous analyses predominantly included discharge medications and did not explore the effect of the drug exposure patterns. We analyzed all-cause death, nonfatal myocardial infarction, repeat revascularization, and major adverse cardiovascular events (MACE) in a cohort of 23,200 post-PCI patients (January 2003 to December 2008) using a multivariate adjusted Cox model and propensity-matched case-control analysis. The adjusted hazard ratio for MACE on PPI according to the exposure patterns of clopidogrel after PCI for 6 years was 1.24 (95% confidence interval [CI] 1.11 to 1.38) and 1.12 (95% CI 1.03 to 1.22) for “continuous” (consistent clopidogrel with or without PPIs) and “switched” (clopidogrel with or without varying PPIs) respectively. However, the propensity score adjusted odds ratios for MACE on PPI use was 0.97 (95% CI 0.65 to 1.44) for “continuous” and 1.04 (95% CI 0.87 to 1.25) for “switched.” Moreover, in the first year after PCI, the use of “rescue” (≤30 days before MACE) nitroglycerin was greater in the patients taking clopidogrel and PPIs than in those taking clopidogrel alone, as was the overall use of rescue PPIs (p <0.001). In conclusion, PPI use in clopidogrel-treated post-PCI patients was not associated with an increased risk of MACE after controlling for the confounding effect of PPI use with propensity matching. A potential for the misdiagnosis of angina symptoms and rescue use of nitroglycerin and PPIs in post-PCI patients exists, a finding that might have confounded previous observational analyses.
Dual antiplatelet therapy with aspirin and clopidogrel has emerged as the cornerstone of therapy for patients with acute coronary syndromes (ACS) who have undergone percutaneous coronary intervention (PCI). The increased risk of gastrointestinal bleeding and data supporting the reduction of gastrointestinal bleeding with proton pump inhibitor (PPI) use have resulted in the widespread prescription of PPIs to patients receiving dual antiplatelet therapy. Given the common metabolic pathway for clopidogrel activation and PPI metabolism mediated by hepatic cytochrome P450 isoenzymes, primarily cytochrome P2C19, the potential exists for competitive inhibition of clopidogrel activation from a prodrug to its active thiol metabolite. This competitive inhibition of clopidogrel activation by PPIs is essentially a pharmacokinetic phenomenon, leading to the generation of reduced levels of the pharmacodynamically active clopidogrel metabolite. These concerns have been substantiated by numerous ex vivo studies and form the basis underlying the concern regarding the attenuation of clopidogrel effects on platelet aggregation. Most observational studies, with the exception of a few performed using propensity score adjustments, have reported an adverse association with the concomitant use of PPIs in clopidogrel-treated patients. These concerns have not been supported by analyses from randomized clinical trials ; moreover, few studies have addressed this concern specifically in post-PCI patients. The fundamental limitation of all current observational studies has been their reliance on discharge prescriptions, without accounting for the varying drug exposure patterns during the follow-up period, because patients often have gaps in therapy. In the present study, we report the findings from an outcomes analysis of post-PCI patients treated with coronary stent implants according to their postdischarge drug exposure patterns using data from a large Veterans Affairs Pharmacy Benefits Management database and the National Patient Care Database.
Methods
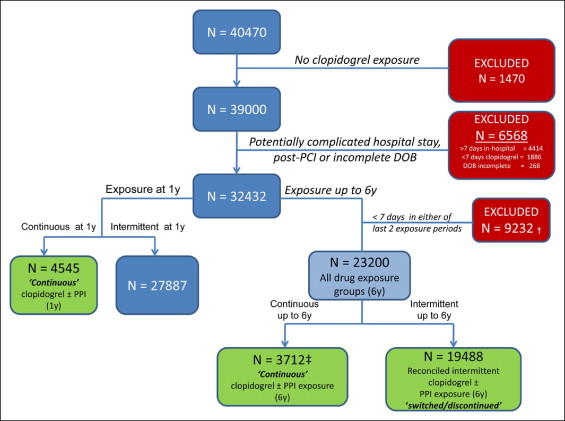
Of 40,470 patients identified from the Veterans Affairs Pharmacy Benefits Management database and National Patient Care Database from January 2003 to December 2008, who had undergone PCI with coronary stent implantation, we selected a study cohort of 23,200 patients. These 23,200 patients had been discharged with clopidogrel after an uncomplicated index PCI (within 7 days), received an outpatient prescription for clopidogrel, and had complete demographic and prescription refill data. The prescribed PPIs included esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole. The prescription drug use of patients and refill data were obtained from the Veterans Affairs Pharmacy Benefits Management database. The baseline characteristics and stent type were recorded. The outcomes analysis was done using the International Classification of Diseases, Ninth Revision , discharge diagnosis and procedure codes available from the National Patient Care Database associated with each patient encounter ( Appendix 1 ). The study cohort selection is outlined in Figure 1 . The patients were categorized according to the consistency and duration of the clopidogrel and PPI exposure patterns using the pharmacy prescription and refill data into exclusive groups. This method is superior to patient self-reported medication use. Daily exposure to clopidogrel and PPIs was derived from the dispensing records using the prescription release dates and days of supply. The prescription release dates were adjusted to not allow double-dosing if the prescriptions had been refilled early. The patients were considered to be taking clopidogrel unless a gap in exposure of >7 consecutive days was noted. Likewise, patients were considered to be taking PPIs unless a gap of >3 consecutive days was noted. Patients with a consistent and uninterrupted exposure to clopidogrel who either consistently used or consistently did not use PPI were included in the “continuous” exposure group. Those with consistent clopidogrel exposure but interrupted PPI exposure during the follow-up period were included in the “switched” group, and patients who discontinued clopidogrel were included in the “discontinued” group. A daily reconciliation of exposure scenarios was performed for each patient. Patients were censored from the study after they had experienced an outcome (e.g., death, death or myocardial infarction, repeat revascularization, major adverse cardiovascular events [MACE]) or had reached the end of the study period. The patients who had received locally filled prescriptions of nitroglycerin and PPIs within 30 days of a MACE or the end of the study (if event free) were evaluated as having received “rescue” medication. All rescue nitroglycerin and PPI prescriptions were filled at local Veterans Affairs (VA) facilities, following a provider prescription collected at the local VA pharmacy. Prescription of an alternate or second PPI to clopidogrel and PPI-treated patients was also considered rescue medication if filled locally within 30 days of MACE or the end of the study. Aspirin use was obtained from the VA pharmacy prescription records for 83.1% of the patients. For the remaining population, it was confirmed by a review of a random sample of VA North Texas Health Care System individual patient medication records of recipients of non-VA aspirin. This method accounted for aspirin use in post-PCI patients taking non-VA aspirin, given the lower cost of over-the-counter aspirin and was used in a previous analysis. The prespecified primary study end point was MACE, a composite of all-cause death, nonfatal myocardial infarction, or the need for repeat revascularization. We also recorded and analyzed the individual components of the MACE outcomes (i.e., all-cause death, all-cause death or nonfatal myocardial infarction, and repeat revascularization). The institutional review board of the VA North Texas Health Care System approved the study, and all data were analyzed in aggregate, without patient identifiers.
The frequencies of the outcomes among the continuous, switched, and discontinued groups by PPI use were compared using 2 by 2 cross-tabulation table chi-square tests, with Bonferroni’s correction for multiple comparisons. No PPI use was the reference group in the calculation of the 2 by 2 contingency table statistics. Two analytic strategies were developed to evaluate the association between PPI use and outcomes, after adjustment for confounders and selection bias: multivariate adjusted Cox regression analysis and propensity score adjustment. In the first model, multivariate-adjusted Cox regression analyses were performed using the outcomes as dependent variables; the primary independent variable was PPI use, controlling for the covariates. These covariates included age, history of hypertension, hyperlipidemia, diabetes mellitus, tobacco use, congestive heart failure, chronic kidney disease, previous myocardial infarction, previous PCI, previous coronary artery bypass graft surgery, ACS, and index PCI stent type (drug-eluting stent or bare metal stent).
The propensity scores for PPI use were calculated as previously described. The individual probabilities for PPI use were calculated according to logistic regression analysis and were retained as a variable for use in matching for the case-control propensity analyses. The “cases” (those with an outcome) were matched to the “controls” (those who were event free) by the calculated propensities of PPI use separately for each outcome (e.g., death, death or myocardial infarction, repeat revascularization, and MACE). The maximum number of significant digits for the probabilities was 10, and the minimum was 3. Matching was halted if a case could not be matched to a control for ≥3 significant digits. This “greedy” matching algorithm has been previously reported. After propensity score matching, the frequency of PPI exposure in the cases versus controls using the odds ratio (OR) was calculated. For all analyses, statistical significance was set, a priori, at p <0.05. An increased frequency of PPI use among cases (e.g., the patients with death, death or myocardial infarction, repeat revascularization, or MACE) compared to the controls (event-free patients) indicated an increased odds of a given outcome associated with PPI use. All analyses were performed using Statistical Analysis Systems, version 9.1, 2009 (SAS Institute, Cary, North Carolina).
Results
The baseline characteristics of the 23,200 patients in the study cohort, stratified by group (continuous, switched, and discontinued) during the 6-year period, are listed in Table 1 . More than 63.0% of the patients received a drug-eluting stent, mostly for ACS (91.1%). Of the 6,030 MACE, 56.0% occurred in the first year after the index PCI. The median interval to MACE for patients taking clopidogrel with PPIs and without PPIs was 3.5 and 2.1 months, respectively. Given that most MACE occurred in the first year after the index PCI, we conducted an additional analysis of the 4,545 patients in the “continuous” exposure group within the first year after their index PCI. The baseline characteristics of this group are listed in Table 2 . The patients with greater exposure to clopidogrel overall (“continuous” and “switched”) had significantly more co-morbid conditions, such as diabetes mellitus, chronic kidney disease, ACS presentation, previous myocardial infarction, previous coronary artery bypass grafting, and greater use of drug-eluting stents during the index PCI than did the clopidogrel-discontinued patients (discontinued group; Table 1 ). Similarly, patients treated concomitantly with a PPI in the first year after PCI also had significantly more co-morbidities ( Table 2 ). Omeprazole was the most frequently used PPI (88.9%) and had been predominantly prescribed for gastroesophageal reflux disease (60.0%).
| Variable | All Patients (n=23,200) | Drug Exposure Groups | ||
|---|---|---|---|---|
| Continuous (n = 3,712) | Switched (n = 7,772) | Discontinued (n = 11,716) | ||
| Mean age (years) | 63.6 ± 10.0 | 64.3 ± 10.0 | 63.7 ± 9.9 | 63.4 ± 10.1 |
| Men | 98.3% | 98.2% | 98.4% | 98.2% |
| Race | ||||
| White | 51.6% | 50.7% | 53.7% | 50.7% |
| Black | 7.7% | 7.1% | 7.1% | 8.3% |
| Hispanic | 3.9% | 3.8% | 4.2% | 3.7% |
| Other/unknown | 36.8% | 38.5% | 35.0% | 37.2% |
| Hypertension | 89.7% | 91.2% ⁎ | 91.3% ⁎ | 88.1% |
| Diabetes mellitus | 45.8% | 50.5% ⁎ | 48.3% ⁎ | 42.6% |
| Hyperlipidemia | 86.2% | 88.6% | 89.4% | 83.3% |
| Tobacco abuse | 47.8% | 44.4% | 57.1% ⁎ | 42.7% |
| Chronic kidney disease | 6.4% | 9.4% ⁎ | 7.4% ⁎ | 4.8% |
| Previous percutaneous coronary intervention | 14.4% | 16.6% ⁎ | 17.3% ⁎ | 11.8% |
| Previous coronary artery bypass graft surgery | 21.9% | 24.4% ⁎ | 25.9% ⁎ | 18.5% |
| Previous myocardial infarction | 19.2% | 20.5% ⁎ | 21.2% ⁎ | 17.5% |
| Acute coronary syndrome presentation | 91.1% | 94.9% ⁎ | 91.7% | 89.5% |
| Heart failure | 23.0% | 25.2% ⁎ | 23.9% | 21.6% |
| Index drug-eluting stent implantation | 63.5% | 70.0% ⁎ | 58.3% | 68.2% |
| Index bare metal stent implantation | 36.5% | 30.0% | 41.7% | 31.8% |
⁎ p < 0.05 for continuous and/or switched groups compared to discontinued group.
| Variable | Study Cohort (n = 4,545) | Clopidogrel Only (n = 3,678) | Clopidogrel Plus PPI Use (n = 867) | p Value |
|---|---|---|---|---|
| Age (years) | 63.9 ± 9.9 | 63.8 ± 9.9 | 64.5 ± 10.3 | 0.060 |
| Men | 98.3% | 98.3% | 98.2% | 0.990 |
| Race | ||||
| White | 53.6% | 51.7% | 61.8% | <0.001 ⁎ |
| Black | 6.2% | 5.9% | 7.2% | 0.182 |
| Hispanic | 3.6% | 3.6% | 3.6% | 1.000 |
| Other/unknown | 36.6% | 38.8% | 27.4% | <0.001 ⁎ |
| Hypertension | 89.5% | 88.9% | 92.4% | 0.003 |
| Diabetes mellitus | 46.3% | 45.1% | 51.4% | <0.001 ⁎ |
| Hyperlipidemia | 87.4% | 86.9% | 89.6% | 0.032 |
| Tobacco abuse | 39.6% | 39.5% | 40.0% | 0.787 |
| Chronic kidney disease | 4.1% | 3.6% | 6.3% | <0.001 ⁎ |
| Previous percutaneous coronary intervention | 14.3% | 12.8% | 20.8% | <0.001 ⁎ |
| Previous coronary artery bypass grafting | 22.3% | 20.7% | 29.2% | <0.001 ⁎ |
| Previous myocardial infarction | 19.0% | 17.6% | 24.9% | <0.001 ⁎ |
| Acute coronary syndrome presentation | 88.7% | 88.4% | 89.9% | 0.255 |
| Heart failure | 22.8% | 20.8% | 31.0% | <0.001 ⁎ |
| Index percutaneous coronary intervention | ||||
| Drug-eluting stent | 53.6% | 51.7% | 61.8% | <0.001 ⁎ |
| Bare metal stent | 46.4% | 48.3% | 38.2% | <0.001 ⁎ |
The hazard ratios (HRs) and 95% confidence intervals (CIs) for all prespecified end points (unadjusted and multivariate Cox model adjusted for covariates listed in the “Methods” section), for all drug exposure categories at 1 year (n = 4,545) and ≤6 years (n = 23,200) are listed in Table 3 . The HR of outcomes associated with PPI use overall replicated the results that have drawn concern regarding this drug regimen for patients exposed concomitantly to clopidogrel and a PPI (continuous and switched groups). However, the propensity-matched OR for MACE in the continuous group was 0.92 (95% CI 0.58 to 1.45) within the first year after PCI and 0.97 (95% CI 0.65 to 1.44) ≤6 years in the PPI groups. The propensity-matched OR for MACE in the switched group was 0.98 (95% CI 0.69 to 1.39) within the first year after PCI and 1.04 (95% CI 0.87 to 1.25) ≤6 years in the PPI groups. We did, however, observe an increased propensity-matched risk of MACE in the discontinued group receiving PPIs (OR 1.21, 95% CI 1.01 to 1.44) within 6 years, unlike within the first year after PCI (OR 0.87, 95% CI 0.46 to 1.67; Table 4 ).
| Group | Event Rates ⁎ | 1-y After PCI | ≤6-y After PCI | |||
|---|---|---|---|---|---|---|
| No PPI | PPI | Unadjusted HR (95% CI) | Adjusted HR † (95% CI) | Unadjusted HR (95% CI) | Adjusted HR † (95% CI) | |
| Continuous | ||||||
| Death | 21.4% | 26.8% | 1.37 (1.03–1.82) | 1.16 (0.87–1.55) | 1.48 (1.13–1.19) | 1.32 (1.00–1.73) |
| Death or myocardial infarction | 44.4% | 48.3% | 1.26 (1.07–1.48) | 1.20 (1.02–1.41) | 1.31 (1.12–1.53) | 1.26 (1.08–1.48) |
| Repeat revascularization | 44.1% | 49.5% | 1.11 (0.95–1.29) | 1.18 (1.01–1.30) | 1.16 (1.00–1.35) | 1.22 (1.05–1.42) |
| Major adverse cardiovascular events | 68.9% | 73.9% | 1.18 (1.05–1.31) | 1.19 (1.06–1.33) | 1.23 (1.10–1.37) | 1.24 (1.11–1.38) |
| Switched | ||||||
| Death | 17.5% | 17.2% | 1.33 (1.03–1.71) | 1.25 (0.97–1.61) | 1.29 (1.09–1.53) | 1.26 (1.06–1.49) |
| Death or myocardial infarction | 28.2% | 32.4% | 1.25 (1.05–1.50) | 1.19 (0.99–1.42) | 1.19 (1.05–1.34) | 1.14 (1.01–1.29) |
| Repeat revascularization | 25.4% | 27.5% | 0.99 (0.83–1.18) | 1.01 (0.84–1.21) | 1.01 (0.97–1.25) | 1.09 (0.96–1.24) |
| Major adverse cardiovascular events | 46.4% | 51.0% | 1.11 (0.98–1.26) | 1.10 (0.97–1.25) | 1.14 (1.05–1.25) | 1.12 (1.03–1.22) |
| Discontinued | ||||||
| Death | 47.9% | 23.7% | 1.09 (0.83–1.44) | 1.00 (0.76–1.33) | 1.19 (1.03–1.38) | 1.11 (0.96–1.29) |
| Death or myocardial infarction | 56.4% | 35.4% | 0.93 (0.75–1.15) | 0.88 (0.71–1.09) | 1.07 (0.95–1.20) | 1.00 (0.89–1.13) |
| Repeat revascularization | 19.6% | 26.3% | 0.93 (0.72–1.18) | 0.96 (0.75–1.23) | 1.08 (0.92–1.27) | 1.06 (0.92–1.27) |
| Major adverse cardiovascular events | 65.0% | 52.4% | 0.93 (0.79–1.09) | 0.91 (0.77–1.07) | 1.14 (1.05–1.25) | 1.03 (0.94–1.13) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


