Endothelin (ET) is involved in the etiopathogenesis of peripheral arterial disease (PAD). We hypothesized that ET antagonism might improve the endothelial function, inflammatory status, and symptoms in PAD. This pilot randomized clinical trial was designed to determine the clinical efficacy, pleiotropic effects, and safety of dual ET-receptor antagonist bosentan in Hispanic patients with PAD presenting intermittent claudication. The Bosentan Population-Based Randomized Trial for Clinical and Endothelial Function Assessment on Endothelin Antagonism Therapy was a 12-month, randomized, controlled, parallel-group, double-blind, proof-of-concept pilot study evaluating the effect of bosentan on absolute claudication distance (primary efficacy end point), flow-mediated arterial dilation, and C-reactive protein levels (primary pleiotropic end points) in patients with PAD with Rutherford category 1 to 2 of recent diagnosis. Secondary end points included ankle-brachial index, subjective claudication distance, and safety. Of the 629 screened subjects, 56 patients were randomized 1:1 to receive bosentan for 12 weeks (n = 27) or placebo (n = 29). Six months after the initiation, a significant treatment effect in flow-mediated arterial dilation of 2.43 ± 0.3% (95% CI 1.75 to 3.12; p = 0.001), absolute claudication distance of 283 ± 23 m (95% CI 202 to 366; p = 0.01), ankle-brachial index of 0.16 ± 0.03 (95% CI 0.09 to 0.23; p = 0.001), and a decrease in C-reactive protein levels of −2.0 ± 0.5 mg/L (95% CI −2.8 to −1.1; p = 0.02) were observed in the bosentan-treated group compared to the control group. No severe adverse effects were found in the bosentan group. In conclusion, in Hispanic patients with intermittent claudication, bosentan was well tolerated and improved endothelial function and claudication distance as well as inflammatory and hemodynamic states.
Recent studies have suggested that endothelin (ET) may play an important role in the alteration of the endothelial function at the onset of peripheral arterial disease (PAD). Likewise, a significant correlation has been shown between plasma ET levels and the number of obstructive arterial atherosclerotic lesions, clinical severity, and endothelial function impairment in such patients. Accordingly, the ET pathway may represent an important target for PAD treatment and prevention through pharmacologic interventions. Bosentan is an orally active dual (ETa and ETb) ET-receptor antagonist that possesses antifibrotic, anti-inflammatory, and antiproliferative properties and selectively vasodilates the diseased vessels without sympathetic reflex activation. However, the effects of bosentan on the vascular function in PAD have not been well researched so far. Meanwhile, previous experiences in Hispanics have evidenced a beneficial effect of bosentan in other forms of peripheral vasculopathy, namely Buerger’s disease. The Bosentan Population-Based Randomized Trial for Clinical and Endothelial Function Assessment on Endothelin Antagonist Therapy (CLAU) was the first pilot trial designed to assess the effects of bosentan on absolute claudication distance (ACD; primary efficacy end point) and flow-mediated arterial vasodilation (FMAD) and C-reactive protein (CRP) levels (primary pleiotropic end points) in Hispanic patients with PAD intermittent claudication. Secondary end points included ankle-brachial index (ABI) and self-reported claudication distance.
Methods
CLAU was a 12-month, randomized, controlled, parallel-group, double-blind, proof-of-concept pilot clinical trial conducted in Spain on a study population of Hispanic male patients with mild-to-moderate claudication (Rutherford category 1 to 2). The study was designed according to the recommendations of the Society for Vascular Surgery Ad Hoc Committee on Clinical Research. The trial protocol was approved by the local investigation ethics committee and was conducted according to the most recent amendments to the Declaration of Helsinki and in adherence to good clinical practice guidelines. Written informed consent was obtained from all patients before enrollment.The study population consisted of Hispanic men aged between 50 and 60 years with incipient stable PAD intermittent claudication (symptoms present for <6 months and not have significantly changed within the previous 3 months). Diagnosis of mild-to-moderate PAD was established by noninvasive vascular hemodynamic testing (ABI <0.90 at rest, standardized measuring) and treadmill test resulting in an ACD between 100 and 500 m associated with postexercise decrease in ABI >20%. Variability of <20% in the ACD in 2 consecutive treadmill tests spaced 2 weeks apart were required for inclusion.
To control any potential confounding factor and due to the proof-of-concept purpose of the study, active smokers or those who quit smoking no earlier than 2 years previous the screening visit were excluded to avoid any possible bias regarding to the potential changes in the smoking habits of those patients during the study. Patients with diabetes were also excluded for claudication distance (because of the diabetic peripheral neuropathy) and hemodynamic assessment difficulties. Patients with a history of coronary ischemic disease and/or stroke were also deemed ineligible. Antiplatelet agents, antihypertension agents, including angiotensin-converting enzyme (ACE) inhibitors, β blockers, angiotensin receptor blockers (ARBs) or calcium channel blockers, and statins were the only allowed concomitant treatment. Medication usages were kept constant throughout the study.
Patients with severe disabling intermittent claudication (Rutherford category 3, <100 m) and critical limb ischemia, manifested by ischemic rest pain, ischemic ulcers, or gangrene (Rutherford category 4 to 6) were excluded from the study. Other major exclusion criteria were previous open or endovascular lower limb revascularization, the history of lumbar sympathectomy, or any formal lower limb revascularization indication criteria. Other conditions that deemed a patient ineligible for inclusion were uncontrolled hypertension, inability to complete the treadmill test for a reason other than ischemic intermittent claudication, previous deep venous thrombosis, other severe concomitant diseases, obesity (body mass index ≥30), and/or substance abuse (other than tobacco, including alcohol).
Eligible patients with a stable treadmill walking performance after 2 weeks were randomized 1:1 to receive bosentan for 12 weeks or placebo, along with optimal medical care (OMC), respectively. In addition to their background OMC for PAD, the experimental group received bosentan (Tacleer; Actelion, Allschwill, Basel, Switzerland) 62.5 mg B.I.D. per oral during the first 4 weeks of the treatment phase to closely control safety issues and 125 mg B.I.D. in the next 8 weeks. The OMC, according to American Heart Association (AHA)/American Stroke Association Guidelines for the management of patients with PAD and Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) II recommendations consisted of: antiplatelet therapy (100 mg/day aspirin or 75 mg/day clopidogrel), statin therapy, and antihypertensive treatment (ACE inhibitors and/or ARBs and/or calcium channel blockers and/or β blockers), and included advice about the use of home exercise and diet in the form of standardized verbal instructions, meeting AHA/TASC II recommendations.
Patients were randomly assigned by computer generation ( http://ww.randomisation.com ) to treatment groups matched on age and ACE inhibitors, ARBs, β blockers, and statins individual intake. Research staff recruiting or treating the patients was not involved in data collection or analysis processes. Treatment allocation was fully concealed from all other researchers and participants. Unblinding occurred after statistical analysis was completed.
Clinical examination, walking treadmill test, FMAD, ABI, Walking Impairment Questionnaire (WIQ), and plasma determinations were assessed periodically at baseline and at weeks 4, 12, 28, and 52.
Walking standardized treadmill test with constant speed of 3.2 km/h and incline of 12.5% were performed in all included patients. The treadmill test was only considered valid whether claudication symptoms were the reason by which the subject had to stop walking. The ACD was defined as the maximum distance walked at which ischemic pain forced the patient to stop on standardized treadmill test.
Treatment compliance, adverse events, concomitant medications, hepatic function, renal function, and hematology values were monitored throughout the study.
FMAD was used as a surrogate measure of endothelial function and performed according to the American College of Cardiology/AHA guidelines. Briefly, FMAD was determined after visualization of the brachial artery using high-resolution Doppler ultrasound (12 MHz linear-array transducer; Phillips, the Netherlands) over the fold of the elbow in a longitudinal section, 60° transducer angle, with 3 measurements of the diameter between the intima-media interfaces. A pressure cuff above the transducer was inflated to 250 mm Hg for 5 minutes, and the brachial artery diameter measured again at 60s after its release. FMAD (%) was calculated by subtracting the mean of the baseline diameter from the mean of the postischemia diameter, divided by the mean baseline diameter, and expressed as percentage. The coefficient of variation for FMAD in our laboratory is 7.3 ± 5.1% as described previously, meeting standardized method and variability measurement values recommended by AHA/American College of Cardiology.
CRP and ET plasma levels were measured in venous blood samples collected from patients. An automated ultrasensitive immunoassay (Roche Diagnostics, Sant Cugat, Barcelona, Spain) was used for measurement of plasma concentrations of CRP. An enzyme immunoassay with mouse monoclonal anti-ET (Biomedica Gruppe, Vienna, Austria) was used to ET determinations.
The primary end points of this proof-of-concept study were the changes in ACD, FMAD and plasma levels of CRP from baseline to weeks 28 and 52. Secondary end points included change from baseline in ABI and the self-reported variation of claudication distance, as defined in the WIQ, and safety. Variations in plasma levels of ET were monitored throughout the study as biomarker of biological effect of bosentan on endothelium.
Superiority tests were conducted comparing bosentan with control group concerning primary and secondary end points. The null hypothesis was that the mean change in each variable from baseline to weeks 28 and 52 in the bosentan group equals that in the control group. Hypothesis testing the treatment effect of bosentan on each study end points were based on 2-sided 95% CIs using Student distribution. Demographics/baseline data and end points outcomes were presented as mean ± SD. Comparisons between groups were made using the Mann–Whitney test for independent variables.
Because there was a lack of existing data for determining the potential benefits of bosentan in improving walking distance, a review of the study was conducted to define the exact effect of bosentan on the levels of ET and, in turn, the relation of ET levels with symptoms of PAD. Assuming that the expected difference in mean changes of FMAD and CRP levels is 0% and the common SD is 30.0% in a sample of 56 evaluable patients randomized 1:1 to bosentan and control, respectively, a 2-group 0.05 2-sided t test had 84% power to reject the null hypothesis in favor of the alternative hypothesis.
Owing to the “placebo effect” on walking ability observed in trials with patients with intermittent claudication, the clinically relevant alternative in ACD was a difference of 50 m between the treatment groups. With the assumption that the data were from approximately normal distributions with a SD ranged from 10% to 30%, the planned 56 patients randomized 1:1 provided 82% power to detect the previously mentioned difference when the data were analyzed by means of Student t test 2-sided 0.05 significance level.
Results
Figure 1 depicts the flow of participants throughout the entire study. Of the 629 patients screened for enrollment, 56 patients were included in the study. A total of 510 patients (81%) did not meet the stringent criteria for inclusion (largely because of the restrictive co-morbidities profile stipulated in the protocol). No patient discontinued the assigned treatment or was lost to follow-up.
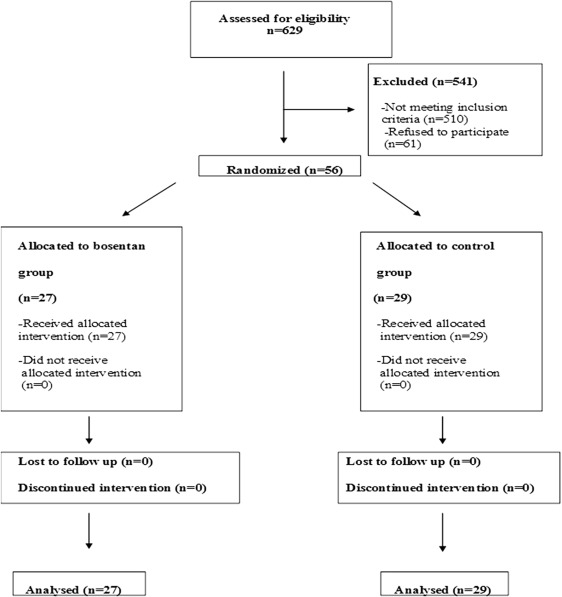
Table 1 summarizes demographic characteristics and baseline values by treatment group in the study per-protocol population. The groups were well matched with respect to demographic features, cardiovascular risk factors, and concomitant medical therapy because of the highly restrictive inclusion criteria and randomization method. Femoral popliteal was the main anatomic lesion level in all patients. A total of 17 patients (20%) were demonstrated to present associated lesions in the aortoiliac sector. All patients were Hispanic Caucasian from a Spanish population.
| Variable | Bosentan Group (n = 27) | Control Group (n = 29) | p |
|---|---|---|---|
| Age (years), Mean±SD | 57.9±1.4 | 57.3±1.3 | 0.88 |
| Weight (kg), Mean±SD | 77.6±9.2 | 76.5±9.8 | 0.83 |
| BMI (kg/m2), Mean±SD | 27.4±2.8 | 26.9±3.3 | 0.78 |
| Hypertension | 27 (100%) | 29 (100%) | 1 |
| Hyperlipemia | 23 (85%) | 25 (85%) | 0.99 |
| Triglycerides(mg/dL), Mean±SD | 163±24 | 169±29 | 0.77 |
| Total Cholesterol(mg/dL), Mean±SD | 195±43 | 196±47 | 0.73 |
| ABI, Mean±SD | 0.67±0.07 | 0.68±0.08 | 0.82 |
| ACD (m), Mean±SD | 427±63 | 423±59 | 0.81 |
| FMAD (%),Mean±SD | 3.5±0.8 | 3.25±0.7 | 0.63 |
| ET(mmol*l -1 ), Mean±SD | 1.36±0.29 | 1.65±0.28 | 0.61 |
| CRP (mg*dl -1 ), Mean±SD | 3.69±1.3 | 3.95±1.1 | 0.79 |
| Statins | 23 (85%) | 25 (85%) | 0.99 |
| ACE inhibitors | 22 (81%) | 23 (81%) | 0.99 |
| ARB | 5 (18%) | 5 (17%) | 0.99 |
| Calcium channel blockers | 5 (18%) | 5 (17%) | 0.99 |
| Beta-blockers | 2 (7%) | 2 (7%) | 0.99 |
Main results of primary and secondary end points are summarized in Tables 2 and 3 . A statistically significant treatment effect in the primary end point ACD was found in the bosentan-treated group at both weeks 28 and 52 compared to controls ( Tables 2 and 3 ). Moreover, it is worthy to be noted that patients in the bosentan group experienced a significant improvement in the ACD, reaching a peak increase at 6th month after randomization with no return to pretreatment ACD values after 12 months ( Supplementary Figure 1 ).
| Variable | Change From Baseline | Treatment Effect | p | |
|---|---|---|---|---|
| Bosentan (n = 27) | Control (n = 29) | (Bosentan-Control) | ||
| ACD (m), Mean±SD | 511±31 | 228±17 | 283±23 | 0.01 |
| FMAD (%),Mean±SD | 2.01±0.4 | -0.42±0.2 | 2.43±0.3 | 0.001 |
| CRP (mg*dl -1 ), Mean±SD | -1.46±0.7 | 0.54±0.2 | -2.0±0.5 | 0.02 |
| ABI, Mean±SD | 0.09±0.05 | -0.07±0.02 | 0.16±0.03 | 0.001 |
| ET(mmol*l -1 ), Mean±SD | -0.13±0.06 | 0.3±0.3 | -0.43±0.1 | 0.86 |
| SCD (m), Mean±SD | 644±103 | 182±93 | 462±87 | 0.05 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


