Lipoprotein(a) [Lp(a)] is an independent risk factor for cardiovascular disease, with limited treatment options. This analysis evaluated the effect of a monoclonal antibody to proprotein convertase subtilisin/kexin 9, alirocumab 150 mg every 2 weeks (Q2W), on Lp(a) levels in pooled data from 3 double-blind, randomized, placebo-controlled, phase 2 studies of 8 or 12 weeks’ duration conducted in patients with hypercholesterolemia on background lipid-lowering therapy (NCT01266876, NCT01288469, and NCT01288443). Data were available for 102 of 108 patients who received alirocumab 150 mg Q2W and 74 of 77 patients who received placebo. Alirocumab resulted in a significant reduction in Lp(a) from baseline compared with placebo (−30.3% vs −0.3%, p <0.0001). Median percentage Lp(a) reductions in the alirocumab group were of a similar magnitude across a range of baseline Lp(a) levels, resulting in greater absolute reductions in Lp(a) in patients with higher baseline levels. Regression analysis indicated that <5% of the variance in the reduction of Lp(a) was explained by the effect of alirocumab on low-density lipoprotein cholesterol. In conclusion, pooled data from 3 phase 2 trials demonstrate substantive reduction in Lp(a) with alirocumab 150 mg Q2W, including patients with baseline Lp(a) >50 mg/dl. Reductions in Lp(a) only weakly correlated with the magnitude of low-density lipoprotein cholesterol lowering.
Elevated lipoprotein(a) [Lp(a)] levels are considered to be an independent risk factor for cardiovascular disease, with risk continuing to increase as levels increase. The 2011 European Atherosclerosis Society/European Society of Cardiology dyslipidemia management guidelines recommend measurement of Lp(a) in high-risk patients or in those with a family history of premature cardiovascular disease, and the European Atherosclerosis Society defines high-risk Lp(a) levels as >50 mg/dl, approximately the eightieth percentile in the Caucasian population. Of the widely prescribed lipid-modifying therapies, only nicotinic acid consistently reduces Lp(a). Despite the potent effect of statins on low-density lipoprotein cholesterol (LDL-C) levels, in general, they show little to no effect on Lp(a) in clinical trials. Alirocumab (formerly SAR236553/REGN727), a highly specific fully human monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9), significantly (p <0.001) reduced LDL-C by up to 72.4% when combined with statin or other lipid-lowering therapy in phase 2 trials and showed a trend to reduce Lp(a), a secondary parameter, across all dosing regimens tested. The objective of the present pooled analysis of alirocumab 150 mg Q2W, a common dose in all phase 2 trials, was to provide, post hoc, a more robust assessment of its effects on Lp(a) and examine the relation with baseline levels and LDL-C reductions.
Methods
Data were pooled from 3 double-blind, randomized, placebo-controlled, phase 2 studies with alirocumab of 8 or 12 weeks’ duration (NCT01288443, NCT01266876, and NCT01288469). The study designs ( Supplementary Figure 1 ) have been previously described. Briefly, in these studies, 352 patients with heterozygous familial hypercholesterolemia or nonfamilial forms of hypercholesterolemia and LDL-C ≥100 mg/dl under background statin or statin plus ezetimibe 10 mg treatment were randomized to alirocumab 50 to 300 mg Q2W or every 4 weeks (n = 275) or placebo (n = 77). One of the studies included uptitration of background atorvastatin from 10 to 80 mg in the placebo arm and 1 of 2 alirocumab arms; in the other alirocumab arm, patients remained at a background atorvastatin dose of 10 mg. This study was included in the pooled analysis because impact of statin uptitration on Lp(a) was expected to be low based on previous studies. This analysis focuses on the dose regimen alirocumab 150 mg Q2W, which was common to all 3 phase 2 studies and was considered to provide consistent robust effects on LDLC ; this is also one of the doses being evaluated in the alirocumab phase 3 clinical trials.
In all phase 2 studies, Lp(a) levels were measured at the same laboratory using rate immunonephelometry (Dade Behring BNII nephelometer; Siemens Healthcare Diagnostics, Deerfield, Illinois). Data on Lp(a) levels at baseline and end of treatment (week 8 or 12 on-treatment value or the last available on-treatment value carried forward) from the modified intention-to-treat populations of the 3 studies were pooled, and percentage changes from baseline for alirocumab 150 mg Q2W and placebo were compared using analysis of covariance with the treatment group and study as fixed effects and baseline Lp(a) as a covariate. p Values associated with these exploratory analyses are provided for descriptive purposes only and were not adjusted for multiplicity. The relation between the percentage changes from baseline in Lp(a) and LDL-C was assessed using linear regression, and the Spearman correlation coefficient was calculated.
Results
Baseline characteristics ( Table 1 ) were well balanced. All patients were receiving background statin therapy, and 11 of 108 patients (10%) in the alirocumab group and 11 of 77 patients (14%) in the placebo group also received ezetimibe 10 mg. Baseline and on-treatment Lp(a) data were available for 102 of the 108 patients who received alirocumab and 74 of the 77 patients who received placebo ( Table 2 ). Thirty-six patients (34%) treated with alirocumab and 25 patients (33%) treated with placebo had baseline Lp(a) >50 mg/dl, the high-risk cut point proposed by the European Atherosclerosis Society guidelines.
| Mean ± Standard Deviation Unless Otherwise Stated | Placebo (n = 77) | Alirocumab 150 mg Every 2 Weeks (n = 108) |
|---|---|---|
| Age (years) | 54 ± 9 | 58 ± 10 |
| Males, n (%) | 38 (49%) | 47 (44%) |
| White or European American, n (%) | 65 (84%) | 96 (89%) |
| Black or African American, n (%) | 10 (13%) | 12 (11%) |
| Other race, n (%) | 2 (3%) | 0 |
| Body mass index (kilograms per meter 2 ) | 29 ± 5 | 29 ± 5 |
| Low-density lipoprotein cholesterol (milligrams per deciliter) | 131 ± 28 | 127 ± 25 |
| Total cholesterol (milligrams per deciliter) | 211 ± 32 | 208 ± 31 |
| High-density lipoprotein cholesterol (milligrams per deciliter) | 52 ± 14 | 54 ± 15 |
| Non-high-density lipoprotein cholesterol (milligrams per deciliter) | 159 ± 31 | 154 ± 31 |
| Triglycerides, median (interquartile range) (milligrams per deciliter) | 123 (92–174) | 124 (88–169) |
| Apolipoprotein B ∗ (milligrams per deciliter) | 109 ± 23 | 108 ± 24 |
∗ n = 76 and n = 107 patients randomized to placebo and alirocumab 150 mg Q2W, respectively, had apolipoprotein B data available at baseline.
| Variable | Placebo (n = 74) | Alirocumab 150 mg Every 2 Weeks (n = 102) |
|---|---|---|
| Lipoprotein(a), median (interquartile range) (milligrams per deciliter) | 19 (6–77) | 30 (8–70) |
| Range, milligrams per deciliter | 2–299 | 2–181 |
| Patients subdivided by baseline lipoprotein(a) | ||
| ≤50 mg/dl, n (%) | 49 (66%) | 68 (67%) |
| >50 mg/dl, n (%) | 25 (34%) | 36 (35%) |
∗ Patients with Lp(a) data available at baseline and end of treatment (week 8/12 on-treatment value or the last available on-treatment value carried forward).
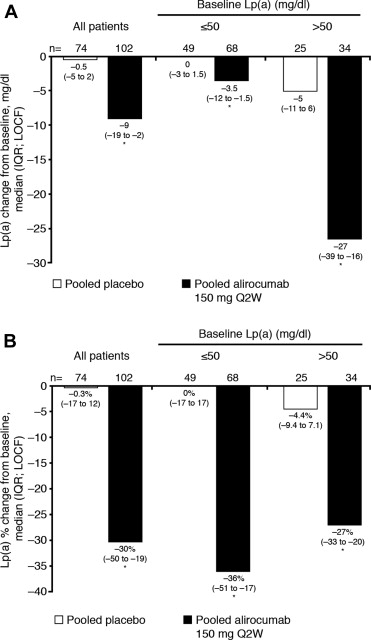
Absolute and percentage median reductions from baseline in Lp(a) are shown in Figure 1 . The median absolute reductions in Lp(a) from baseline were substantially greater in patients with a higher baseline Lp(a) level ( Figure 1 ). Reductions in Lp(a) were only weakly correlated with the magnitude of LDL-C lowering (Spearman correlation coefficient, 0.2236; Supplementary Figure 2 ). Similar results were observed using a regression analysis comparing actual achieved LDL-C levels and percentage reduction in Lp(a) ( Supplementary Figure 2 ). The percentage reductions in Lp(a) from baseline with alirocumab were consistent between a pool of the two 12-week studies and a pool of the 2 arms receiving alirocumab in the 8-week study (−28.3% and −32.1%, respectively).
The most common treatment-emergent adverse event in the alirocumab phase 2 trials was injection site reaction, typically episodic and of mild intensity and short duration; 5 serious adverse events occurred in 4 patients (1.5%) who received alirocumab.
Results
Baseline characteristics ( Table 1 ) were well balanced. All patients were receiving background statin therapy, and 11 of 108 patients (10%) in the alirocumab group and 11 of 77 patients (14%) in the placebo group also received ezetimibe 10 mg. Baseline and on-treatment Lp(a) data were available for 102 of the 108 patients who received alirocumab and 74 of the 77 patients who received placebo ( Table 2 ). Thirty-six patients (34%) treated with alirocumab and 25 patients (33%) treated with placebo had baseline Lp(a) >50 mg/dl, the high-risk cut point proposed by the European Atherosclerosis Society guidelines.
| Mean ± Standard Deviation Unless Otherwise Stated | Placebo (n = 77) | Alirocumab 150 mg Every 2 Weeks (n = 108) |
|---|---|---|
| Age (years) | 54 ± 9 | 58 ± 10 |
| Males, n (%) | 38 (49%) | 47 (44%) |
| White or European American, n (%) | 65 (84%) | 96 (89%) |
| Black or African American, n (%) | 10 (13%) | 12 (11%) |
| Other race, n (%) | 2 (3%) | 0 |
| Body mass index (kilograms per meter 2 ) | 29 ± 5 | 29 ± 5 |
| Low-density lipoprotein cholesterol (milligrams per deciliter) | 131 ± 28 | 127 ± 25 |
| Total cholesterol (milligrams per deciliter) | 211 ± 32 | 208 ± 31 |
| High-density lipoprotein cholesterol (milligrams per deciliter) | 52 ± 14 | 54 ± 15 |
| Non-high-density lipoprotein cholesterol (milligrams per deciliter) | 159 ± 31 | 154 ± 31 |
| Triglycerides, median (interquartile range) (milligrams per deciliter) | 123 (92–174) | 124 (88–169) |
| Apolipoprotein B ∗ (milligrams per deciliter) | 109 ± 23 | 108 ± 24 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


