Chapter 13 Ebstein’s Anomaly of the Tricuspid Valve
In June 1864, a 19-year-old laborer was admitted to the All-Saints Hospital in Breslau (now Wroclaw), Poland, where he died 8 days later. Wilhelm Ebstein, then assistant physician at All-Saints, performed a postmortem examination the following day and subsequently wrote a scholarly account of the clinical and necropsy findings entitled “On a Very Rare Case of Insufficiency of the Tricuspid Valve Caused by a Severe Congenital Malformation of the Same.”1 Ebstein’s meticulous correlation of pathology with clinical notes and hypotheses of pathophysiology resulted in a landmark publication.2 English translations are available and repay study.3–5
Ebstein’s report was virtually overlooked until 1937 when Yater and Shapiro6 published an account of radiologic and electrocardiographic observations of the anomaly.
In 1949, Tourniaire, Tourniaire, and Tartulier7 diagnosed the malformation in a living subject; and in 1950, Engle and colleagues8 analyzed data from three patients who died of Ebstein’s anomaly and asserted that clinical recognition was possible. In the same year, Reynolds9 published the first English-language report of cases recognized during life. Shortly thereafter, Soloff, Stauffer, and Zatuchni10,11 made the clinical diagnosis in a 34-year-old patient and confirmed the diagnosis at necropsy 6 years later. The anomaly or malformation that Ebstein described occurs in approximately 1 in 20,000 live births,5,12,13 accounts for 0.3% to 0.7% of all cases of congenital heart disease, and represents about 40% of congenital malformations of the tricuspid valve.14
Normal tricuspid leaflets consist of basal attachments to the annulus (right atrioventricular sulcus), peripheral zones into which chordae tendineae insert, and clear zones that lie between the basal attachments and the peripheral zones.15 The semicircular or quadrangular anterior leaflet is the largest of the three.15 The posterior leaflet is scalloped. The septal leaflet attaches chiefly to the ventricular septum as the name indicates, but part of its basal attachment is to the posterior wall of the right ventricle.15 The septal leaflet normally exhibits a slight but distinct apical displacement of its basal attachment compared with the mitral valve: 15 mm in children, and 20 mm in adults.16
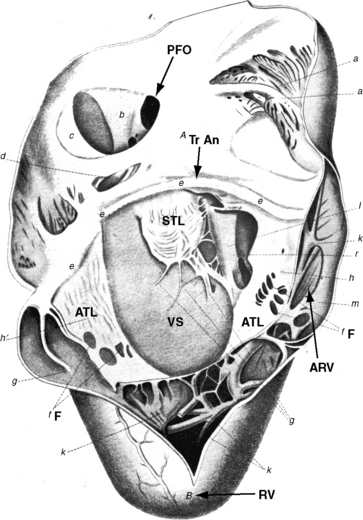
Ebstein wrote, “When we turn our attention to the description of the right ventricle, we see at once an extremely abnormal appearance of the tricuspid valve”1–4 (Figure 13-1). The septal and posterior leaflets do not attach normally to the tricuspid annulus, so the valve orifice is displaced downward into the right ventricular cavity at the junction of the inlet and trabecular components of the right ventricle.16,17
The right side of the heart therefore consists of three morphologic components: the right atrium proper; the inlet portion of the right ventricle, which is thin-walled and functionally integrated with the right atrium; and the trabecular and outlet portion, which constitute the functional right ventricle. The greater the apical displacement of the posterior and septal leaflets, the larger the atrialized right ventricle and the smaller the functional right ventricle (Figure 13-2). Downward displacement of the septal tricuspid leaflet is associated with discontinuity of the central fibrous body and the septal atrioventricular ring, which creates a potential substrate for accessory pathways and preexcitation. The leaflets and tensile apparatus of the tricuspid valve are thought to be formed chiefly by delamination of the inner layers of the inlet zone of the right ventricle. When the tricuspid leaflets are displaced downward, delamination fails to occur.
Morbid anatomy encompasses a broad range of severity,17,18 but certain features are relatively constant.19,20 The anterior leaflet is almost always attached to the atrioventricular junction. A salient anatomic feature is the level of the hinge points of the septal and posterior leaflets, which are characterized by apical displacement of their basal attachments,16 adherence to the underlying myocardium,17 and impaired movement because of short chordae tendineae and nodular fibrotic thickening (see Figure 13-1). When the septal leaflet is absent, the arrangement of the posterior leaflet distinguishes Ebstein’s anomaly from a congenitally unguarded tricuspid orifice (see subsequent). The anterior leaflet differs appreciably from the septal and posterior leaflets. The basal attachment is at the level of the atrioventricular sulcus (annulus). The large and potentially mobile anterior leaflet contains muscular strands instead of consisting entirely of a fibrous membrane as in the normal tricuspid valve. Mobility is impaired by thickening, modularity, fibrosis, and multiple short chordae.
The displaced insertions of the malformed septal and posterior leaflets allow free communication between the proximal (atrialized) and distal (functional) right ventricle. Occasionally, communication between the atrialized right ventricle and the functional right ventricle is confined to slits or perforations in the anterior tricuspid leaflet, as Ebstein originally described, or the two segments are separated by a muscular partition or shelf (see Figure 13-32) that restricts flow to the commissure between the anterior leaflet and the displaced septal leaflet.21 When the anteromedial commissure is fused and the anterior leaflet is intact, the tricuspid orifice is imperforate.21
The thin-walled atrialized right ventricle is relatively devoid of muscular tissue and is typically dilated, often aneurysmally.22 It expands paradoxically during ventricular systole, thus acting as a passive reservoir that decreases the volume of ejected blood.17 Morphometric analyses of the trabecular and infundibular portions of the functional right ventricle disclose an absolute decrease in the number of myocytes and an increase in fibrosis that are held responsible for infundibular dilation.19
An ostium secundum atrial septal defect is present in over a third of hearts with Ebstein’s malformation, and most of the rest have a patent foramen ovale (see Figures 13-1 and 13-2).23 Ebstein believed that “[r]egurgitation of blood into the right atrium caused its dilatation and prevented complete closure of the valve of the foramen ovale.”1,17 Ebstein’s anomaly of an inverted tricuspid valve in congenitally corrected transposition of the great arteries is dealt with in Chapter 6.
Tricuspid regurgitation from a congenitally unguarded tricuspid orifice24 or tricuspid valve dysplasia differs fundamentally from Ebstein’s anomaly.25 A congenitally unguarded tricuspid orifice is characterized by absence of all three leaflets24 or by a muscular partition or shelf that leaves the atrioventricular orifice unguarded (see Figure 13-32). Congenital dysplasia of the tricuspid valve refers to nodular thickening and rolling of the edges of the leaflets without downward displacement.25
Idiopathic dilation of the right atrium has been reported in children without Ebstein’s anomaly.26,27 Transient tricuspid regurgitation of the newborn has no definable anatomic basis and resolves within a few weeks.28 Uhl’s anomaly is discussed at the end of this chapter.
Abnormalities of the left side of the heart have been reported in 39% of patients with Ebstein’s anomaly17,29 and consist of derangements in left ventricular geometry, impairment of systolic and diastolic function,29,30 and noncompaction.17,31 Superior systolic displacement of the mitral valve (prolapse) occurs because mitral leaflets with normal areas and chordal lengths are housed in a left ventricular cavity that is geometrically altered and reduced in size (see Figure 13-31).19,29,32,33 Depressed systolic function is the result of a combination of abnormal shape, impaired diastolic filling, and increased fibrous content of the free wall and the ventricular septum.19
The physiologic consequences of Ebstein’s anomaly are determined by the morphologic derangement of the tricuspid leaflets, by the hemodynamic burden imposed on an inherently flawed the right ventricle, by left ventricular function, and by atrial rhythm. The tricuspid orifice is typically incompetent, occasionally stenotic, and rarely imperforate. Functional impairment of the right ventricle depends on the severity of tricuspid regurgitation and on the size of the right atrium and atrialized right ventricle relative to the size of the functional right ventricle. The thin-walled atrialized right ventricle is either passive during the cardiac cycle or functions as an aneurysm that expands paradoxically during systole and therefore acts a physiologic impediment (see previous discussion). Exercise intolerance has been ascribed to an inadequate increment in pulmonary blood flow and a fall in systemic arterial oxygen saturation.34,35 Atrial tachyarrhythmias have serious physiologic implications, especially the rapid heart rates associated with accessory pathways (see sections The Electrocardiogram and The History).
The functionally inadequate right ventricle is especially vulnerable in utero and at birth. High neonatal pulmonary vascular resistance increases right ventricular afterload and augments tricuspid regurgitation. Right atrial pressure increases, and a right-to-left shunt is established across a patent foramen ovale or an atrial septal defect. The process is reversed as neonatal pulmonary vascular resistance falls and right ventricular afterload normalizes. The enlarging right atrium becomes sufficiently commodious to accommodate a large volume of regurgitant flow with little or no increase in pressure (Figure 13-5). In older patients, right ventricular filling pressure may increase, provoking a rise in right atrial pressure with reestablishment of the right-to-left interatrial shunt.
Ebstein’s original case was an example of obstruction at the tricuspid orifice (see Figure 13-1).1 He wrote, “The membrane divided the right ventricle into two halves. These halves communicated with each other in two ways: one through the oval opening which leads into the right conus arteriosus, and two through the already described multiple openings in the fenestrated membrane.”1 When the tricuspid orifice is stenotic or imperforate, the elevation of right atrial pressure reflects the degree of obstruction. The A wave is often giant, and a right-to-left interatrial shunt persists after the fall in neonatal pulmonary vascular resistance.
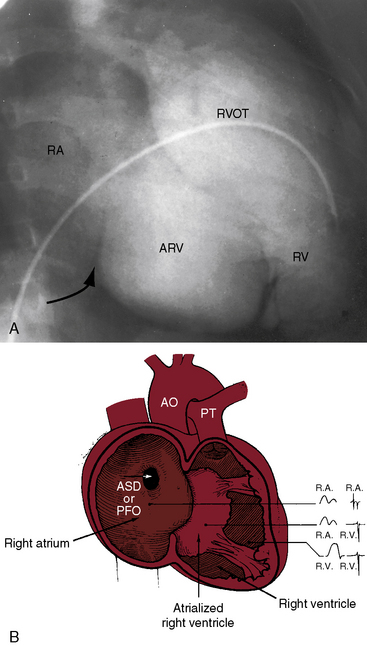
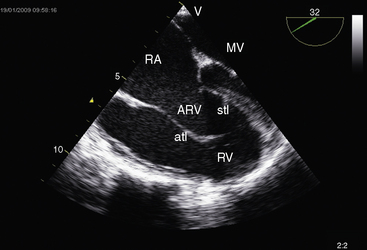
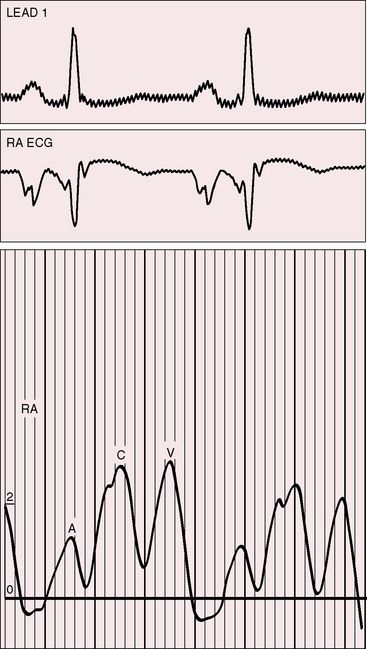
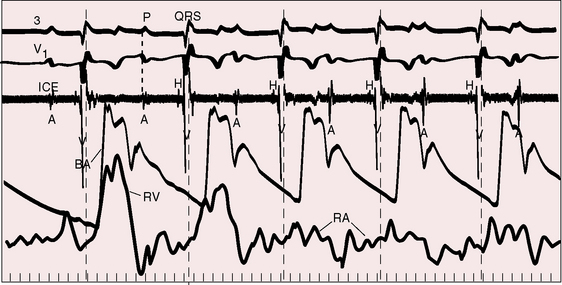
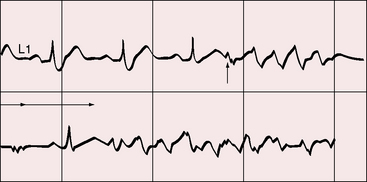
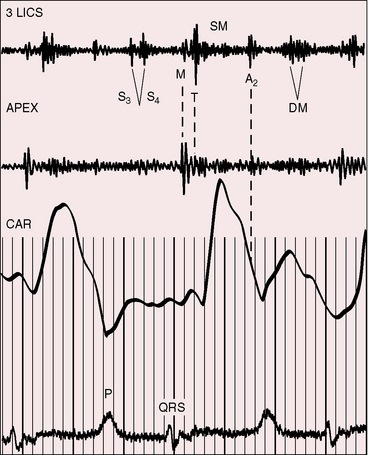
The electromechanical properties of the right atrium, atrialized right ventricle, and functional right ventricle provided the first secure basis for the clinical diagnosis of Ebstein’s anomaly (Figures 13-2 and 13-3).36,37 The right atrium proper generates a right atrial pressure pulse and an intracavitary atrial electrogram. The functional right ventricle generates a right ventricular pressure pulse and a right ventricular intracavitary electrogram. The atrialized right ventricle generates a right ventricular intracavitary electrogram but an atrial pressure pulse (see Figures 13-2 and 13-3). Mechanical stimulation of the atrialized right ventricle provokes a right ventricular electrogram and incurs the risk of triggering ventricular tachycardia.38,39 Clusters of ventricular cardiomyocytes are isolated within a fibrous matrix that prevents spiral/scroll reentrant waves from anchoring.40 When spiral/scroll waves do not anchor, they meander erratically as polymorphic ventricular tachycardia. Accordingly, mechanical stimulation of the atrialized right ventricle does not provoke monomorphic ventricular tachycardia that depends on slow conduction, unidirectional block, and a substrate that permits reentry but instead results in polymorphic ventricular tachycardia.38,40
The geometric configuration and function of the right and left ventricles are closely coupled in Ebstein’s anomaly.29 Leftward displacement of the ventricular septum (see Figure 13-31) reduces the volume of left ventricular diastolic filling and, accordingly, reduces the ejection fraction. Exercise provokes an increase in left ventricular ejection fraction because end-systolic volume decreases with little or no change in end-diastolic volume. The right ventricular free wall contributes feebly if at all to forward flow, which is materially assisted by paradoxical motion of the ventricular septum that functions as part of the right ventricle as in Uhl’s anomaly (see subsequent). In brief, left ventricular function is adversely affected by the diastolic position of the ventricular septum, geometric distortion of the ventricle (see Figure 13-31), reduced end-diastolic volume, paradoxical motion of the ventricular septum, an increase in fibrous tissue, and a decrease in cardiomyocytes in the free wall and septum.19
History
Males and females are equally affected.41–44 Familial Ebstein’s anomaly has been reported,41,43,45–51 and a patient with Ebstein’s anomaly of a right-sided tricuspid valve had a cousin with congenitally corrected transposition of the great arteries and Ebstein’s anomaly of an inverted left–sided atrioventricular valve.41 A novel mutation is held responsible for the genetic syndrome of ventricular preexcitation and conduction system disease of childhood onset,52 but these observations cannot be extrapolated to the general population of patients with preexication or to patients with Ebstein’s anomaly in the absence of preexcitation.
The Danish Registry estimates that the relative risk of Ebstein’s anomaly is increased by 500-fold in offspring exposed to in utero lithium carbonate, which is used for treatment of bipolar disorders.12,53,54
The probability of occurrence of Ebstein’s anomaly in the general population is 1:20,000, so the likelihood of the malformation occurring spontaneously in a pregnant woman taking lithium is 1:20 million.54 Estimates now show that the increased risk incurred with lithium does not exceed 28-fold, which is considerably less than estimates from the Danish Registry.12,53
The clinical course of Ebstein’s anomaly ranges from intrauterine death to asymptomatic survival to late adulthood.43,55–58 The most common presentations are: 1, detection of the anomaly in a routine fetal echocardiogram; 2, neonatal cyanosis; 3, heart failure in infancy; 4, murmur in childhood; and 5, arrhythmias in adolescents and adults.41,57 The outlook for fetal Ebstein’s anomaly has aptly been characterized as appalling.57 Of neonates with Ebstein’s anomaly, 20% to 40% do not survive 1 month, and less than 50% survive to 5 years.17 Fetal hydrops is almost invariably fatal with rare exception.59,60 Neonates not only have high mortality rates but also a significant ongoing risk of morbidity and death.43,57,58 Even if symptoms resolve in the first month of life, the infant may then die suddenly. Transient neonatal cyanosis that recurs a decade or more later is an uncommon but distinctive and usually benign feature of Ebstein’s anomaly.57 A neonatal right-to-left interatrial shunt disappears as pulmonary vascular resistance normalizes; the shunt subsequently reappears as filling pressure rises in the functionally abnormal right ventricle. Tachyarrhythmic sudden death looms as a threat regardless of severity of the anomaly56,61 and is responsible for the decline in survival rate in the fifth decade.56 Wolff-Parkinson-White syndrome in otherwise healthy individuals carries an estimated sudden cardiac death risk of 0.02%,62 but in Ebstein’s anomaly, atrial flutter or fibrillation with accelerated conduction is accompanied by a major increase in the risk of sudden death. Stimulation of the arrhythmogenic atrialized right ventricle initiates polymorphic ventricular tachycardia that promptly degenerates into ventricular fibrillation (Figure 13-4; see previous discussion), and spontaneous ventricular tachycardia/fibrillation looms as a threat. The degree of cyanosis does not necessarily correspond with symptoms, but once cyanosis and symptoms develop, disability tends to be progressive, even in patients who were relatively asymptomatic before adulthood. The onset of chronic atrial fibrillation prefigures death within 5 years.56
Despite qualifications, legendary accounts are found of astonishing longevity in Ebstein’s anomaly, with survivals into the eighth and ninth decades.13,41,43,63,64 Ebstein’s anomaly was discovered at necropsy in a 75-year-old man who as a youth had been a lumberjack working on log booms.65 He was reportedly asymptomatic until his fifties when he was obliged to outrun an irate female bear.65 At necropsy 25 years later, his right atrium was thin-walled and greatly dilated, and the tricuspid valve was characteristically malformed.65 The oldest recorded patient with Ebstein’s anomaly lived to age 85 years and was devoid of cardiac symptoms until age 79 years.66
The chest pain that occasionally occurs with Ebstein’s anomaly is an enigma.41 The pain is retrosternal, epigastric or in the right or left anterior chest, and is sharp, stabbing, or shooting, features that suggest serous surface origin. A fibrinous pericardium has been found at necropsy over the atrialized right ventricle (see section on Auscultation).
Important although less frequent manifestations result from paradoxical emboli or brain abscess. Infective endocarditis is uncommon because regurgitant flow across the malformed tricuspid valve is low velocity with low turbulence. Prophylaxis for the low risk of infective endocarditis is open to question.17 Pregnancy incurs the risks inherent in a functionally inadequate volume-overloaded right ventricle that copes poorly with the additional hemodynamic burden of gestation.67 Paroxysmal atrial tachyarrhythmias are potential hazards during pregnancy, especially the rapid rates associated with accessory pathways. Cyanosis may first become manifest during pregnancy because of a rise in filling pressure in the volume-overloaded right ventricle. Hypoxemia increases the risk of fetal wastage,68 and a right-to-left interatrial shunt incurs a puerperal risk of paradoxical embolization.
Physical appearance
Growth and development are normal in patients who were asymptomatic as neonates and infants. Persistent cyanosis or intermittent exercise-induced cyanosis occurs in more than 50% of cases.41
Jugular venous pulse
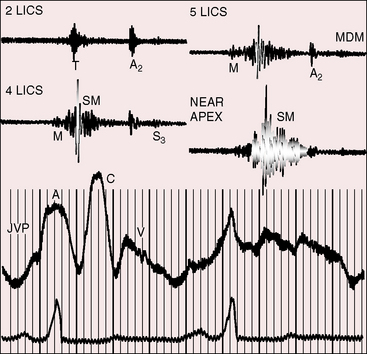
The jugular pulse is normal except for a prominent C wave that coincides with mobility of the anterior tricuspid leaflet (Figures 13-5 and 13-6, first panel). The interval between the jugular A wave and the carotid pulse is often prolonged, reflecting prolongation of the PR interval (see section on The Electrocardiogram). Prominent A waves are seldom seen in the jugular pulse, but atrial contraction sometimes generates presystolic waves in the pulmonary arterial pressure pulse. A stenotic or imperforate tricuspid orifice is accompanied by A waves that may be giant. An attenuated X descent and a systolic venous V wave of tricuspid regurgitation seldom appear in the jugular pulse despite severe regurgitant flow because of the damping effect of the commodious right atrium and the thin-walled toneless atrialized right ventricle and because tricuspid regurgitation is low-pressure and hypokinetic (see Figures 13-5 and 13-6). Right ventricular failure induces a rise in mean jugular venous pressure and a rise in A and V wave crests, but systolic pulsations of the liver are inconspicuous because the right ventricle is hypokinetic.
Precordial movement and palpation
A right ventricular impulse and a tricuspid systolic thrill are reserved for neonates before normalization of pulmonary vascular resistance.41 With this exception, absence of a systolic impulse over the inflow portion of the right ventricle is an important negative sign in the clinical diagnosis of Ebstein’s anomaly.41,69 An undulating rippling motion over the atrialized right ventricle is sometimes seen in older patients. Enlargement of the infundibular portion of the right ventricle is accompanied by a systolic impulse in the third left intercostal space.70 Ebstein described a gentle left ventricular impulse, stating, “… the cardiac apex was visible under the sixth rib somewhat outside the mammary line.”1 Ebstein also percussed the enlarged right atrium: “… the cardiac dullness extended for two centimeters beyond the right border of the sternum at the level of the fourth rib and for three centimeters at the level of the sixth rib, where it merged with the liver dullness which was normal in extent.”1
The initial component of a widely split first heart sound coincides with mitral valve closure, and the second component, which is delayed, coincides with closure of the large anterior tricuspid leaflet (Figures 13-6 through 13-9).69–72 The delay in tricuspid valve closure is not simply the result of complete right bundle branch block and a hypokinetic right ventricle but instead is the result of the large size and increased excursion of the anterior leaflet, which requires longer to reach its fully tensed closed position.72 The increased tension developed by the large anterior leaflet as it reaches the limits of its systolic excursion accounts for the loudness of the tricuspid component of the first heart sound—the sail sound—which is an important auscultatory sign of anterior leaflet mobility (see Figure 13-30).68,71 When a long PR interval softens the mitral component, the first heart sound is then represented by a loud single tricuspid component. Preexcitation of the right ventricle buries the mitral component of the first sound in an early loud tricuspid component (Figure 13-10).73
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree