(1)
Department of Neuroscience, University of Turin Ospedale Molinette, Turin, Italy
Abstract
Isolated cases of dural arteriovenous fistula (DAVF) have been reported by some authors (Verbiest 1951; Obrador and Urquiza 1952). Progressive identification and precise description of this pathology came at the end of the 1960s, especially after selective and super-selective angiographic studies (Hayes 1963; Laine et al. 1963; Newton et al. 1968; Newton and Hoyt 1970; Djindjian et al. 1968, 1973).
Isolated cases of dural arteriovenous fistula (DAVF) have been reported by some authors (Verbiest 1951; Obrador and Urquiza 1952). Progressive identification and precise description of this pathology came at the end of the 1960s, especially after selective and super-selective angiographic studies (Hayes 1963; Laine et al. 1963; Newton et al. 1968; Newton and Hoyt 1970; Djindjian et al. 1968, 1973).
13.1 Incidence
DAVFs account for 10–15 % of all intracranial vascular malformations. Both sexes are affected, but a sexual predominance occurs in some types of DAVF. The fistulas are more frequent among middle-aged and older patients, though younger patients and children can be affected.
13.2 Pathology and Pathogenesis
DAVFs consist of a shunt between dural (meningeal) arteries and sinuses, either directly or mediated by cortical or other sinusal veins. DAVF is considered to be an acquired pathology (Houser et al. 1979; Chaudary et al. 1982). The specific pathogenesis, however, is still debated. Some authors believe (Houser et al. 1979; Mironov 1995) DAVFs are preceded by thrombosis of a sinus. When the lumen recanalizes, microscopic AV shunts, normally present within the wall of the sinus (Kerber and Newton 1973), may enlarge and open into the sinus. Considering, however, that sinus thrombosis is not necessarily associated with DAVF and that DAVFs are not always associated with sinus thrombosis, other authors (Chaloupka and Huddle 1998; Chaloupka et al. 1999) have suggested that the primary cause is angiogenesis within the sinus wall. This leads to the formation of abnormal arteriovenous connections and finally to DAVF. Indeed, abnormal artery–vein connections have been demonstrated in histological studies of resected specimens of sinuses of patients with DAVF (Nishijima et al. 1992; Hamada et al. 1997). The cause of the microfistulas in the sinus wall is not clear. Some authors have shown with studies in animal models that venous hypertension can lead to DAVF formation more frequently in animals with induced hypertension than in those without (Terada et al. 1994; Lawton et al. 1997). In this context, flow changes in the venous sector with possible partial sinus thrombosis, and venous hypertension has been suggested to be at least one of the causes of de novo dural arteriovenous fistula occurring after endovascular occlusion of the brain AVM (Paramasivan et al. 2013). Other studies (Uranishi et al. 1999; Shin et al. 2007a) have demonstrated in resected dural specimens of DAVF patients and in rat models the presence of an angiogenetic growth factor that can participate in the fistula formation. Other authors (Klisch et al. 2005) examined, in the blood of patients with DAVF, the concentration of the endothelial growth factor (VEGF) and the angiopoietic receptor (TIE-2). These factors were increased in the pretreatment phase and decreased after the endovascular treatment.
More difficult to explain are DAVFs located in the dura, close to the sinus, but not draining directly into it. The outflow is characterized by pial veins, which, after a course more or less long, enter a near or distant sinus. In these cases, a nonspecific thrombophlebitis involving the pial veins has been suggested (Djindjian and Merland 1978).
13.3 Clinical Relevance
Many DAVFs remain asymptomatic or have a benign course. In other cases, DAVFs can have a more aggressive course, characterized by cranial nerve palsy, ischemia, hemorrhage, and cognitive disorders. In this context, it has become increasingly clear that the main factor responsible for the symptoms and evolution of DAVFs is the pattern of venous drainage (Lasjaunias et al. 1986b; Halbach et al. 1988; Awad et al. 1990; Awad 1993; Barnwell et al. 1991; Lasjaunias and Rodesch 1993; Cognard et al. 1995; Davies et al. 1996).
13.4 Location
DAVFs can occur anywhere. The most frequent are those involving the transverse–sigmoid sinuses, followed by the cavernous and superior sagittal sinus. Less common are ethmoidal (anterior cranial fossa) and tentorial DAVFs and DAVFs located in the area of the foramen magnum involving different venous channels.
13.5 Diagnosis
DAVFs involve the preexisting vascular structure (dural branches, dural sinuses, pial veins) of the area in which they develop, and a typical repetitive pattern can be expected, corresponding to the site and type of fistula. However, it should be noted that there are many variants concerning the arteries supplying the dura and the venous drainage of the area involved. Furthermore, such variants can be altered by sinus thrombosis, and so it is not surprising that fistulas at the same site can have different angiographic patterns.
A complete angiographic study with examination of the external carotid artery (ECA), internal carotid artery (ICA), and vertebral artery (VA) is essential for the diagnosis. This provides information about all the dural branches involved and the venous drainage. The site, the extension of the DAVF, and, in particular, the type of venous drainage explain the symptoms and offer information about the risk and prognosis of the fistula. Furthermore, a decision can be made about whether the fistula should be treated and, if so, whether surgical or endovascular therapy should be used; in the case of endovascular treatment, the better route—arterial or venous—has to be decided.
13.6 Classification
Castaigne et al. (1976) first distinguished DAVFs draining directly into the sinus from those in which the drainage into the sinus was mediated by a cortical vein. Taking into consideration mainly the type of venous drainage, Djindjian and Merland (1978) made the first classification. This was revised by Cognard et al. (1995) and Borden et al. (1995). Five types of DAVFs can be identified:
1.
Drainage into a main sinus with an anterograde flow.
2.
(a)
Drainage into a main sinus with reflux within the sinus, but not into the pial veins; the latter drain normally into the affected sinus.
(b)
Drainage into the sinus with reflux into the pial veins alone or associated with sinus reflux.
3.
Drainage into the sinus is mediated by the pial veins.
4.
Drainage into the sinus is mediated by the pial veins, which present a large ectasia.
5.
Drainage involves the spinal perimedullary vein.
Type 1 commonly has a benign course. In types 2 and 3, the impaired normal drainage can lead progressively to venous congestion, ischemia, and/or intracranial hypertension. In types 3 and 4, hemorrhage is frequent owing to rupture of the pial vein draining the shunt. Cranial nerve palsy can occur, particularly in DAVF of the cavernous sinus and DAVFs located close to the brainstem. Drainage involving the spinal perimedullary vein can lead to involvement of the cervical spinal cord and brainstem.
13.7 Situations Deserving More Detailed Consideration
1.
DAVFs involving the transverse sinus (TS) and sigmoid sinus (SiS) are the most frequent (Halbach et al. 1987; Awad 1993). In many cases, these belong to type 1 and so can be characterized by a benign course. The only symptom is bruit since the fistula is close to the petrous bone, containing the auditory apparatus. In some cases, the bruit can become very loud and intolerable for the patient, necessitating treatment. Some of these fistulas can change (Piton et al. 1984) and become type 2, with a progressively large reflux in the temporal veins, leading to venous congestion. This is particularly the case when a distal or proximal thrombosis of the sinus occurs. In other less common cases, the sinus can be occluded proximally and distally (isolated sinus). The symptoms in such patients can be very severe, characterized by cognitive disorders, epileptic seizures, and other neurological impairments due to venous congestion or hemorrhage (Naito et al. 2001; Bradac et al. 2002; Kiura et al. 2007). The supplying arteries can vary, but commonly branches of the ECA (occipital, ascending pharyngeal artery, middle meningeal artery) are involved uni- or bilaterally. Meningeal branches of the cavernous portion of the ICA and meningeal branches of the VA can also supply the fistula. Endovascular treatment with an arterial or venous approach is commonly the therapy of choice (Figs. 13.2 and 13.3). Surgery is frequently used in cases of an isolated sinus, even if an endovascular approach has been successfully used in some cases (Fig. 13.1). To this group of DAVFs can be included (Fig. 13.12) also those of the torcular herophili (see also tentorial DAVF). These are very rare fistulas belonging commonly to type 2 or 3. The feeders are the MMA and occipital arteries bilaterally, the meningeal branches of the cavernous portion of the ICA, and meningeal branches from the VA uni- or bilaterally. Pial branches of the cerebellar arteries can also be involved. The torcular herophili is frequently thrombosed, with thrombosis extending to the proximal TS uni- or bilaterally and sometimes to the distal superior sagittal sinus (SSS). The venous drainage is rerouted in the straight sinus, vein of Galen, and then in the basal vein and deep cerebral veins. Venous congestion in the cerebral hemispheres and cerebellar hemispheres is frequent. Intracranial hypertension and hemorrhage are frequent. Surgical excision has been the treatment of choice. Endovascular treatment, with selective catheterization of the feeders, followed by injection of acrylic glue or Onyx, and the venous approach through the SiS–TS when at least one TS is patent, followed by placing of coils, is in many cases a valid and successful alternative (Kirsch et al. 2009; Macdonald et al. 2010).
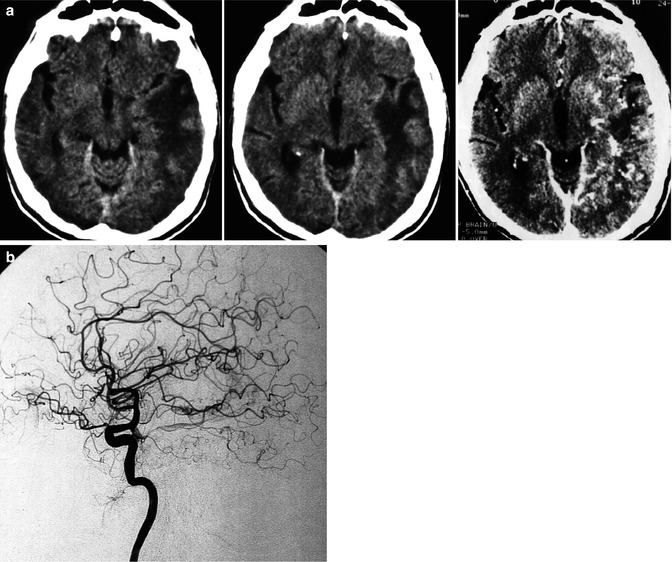
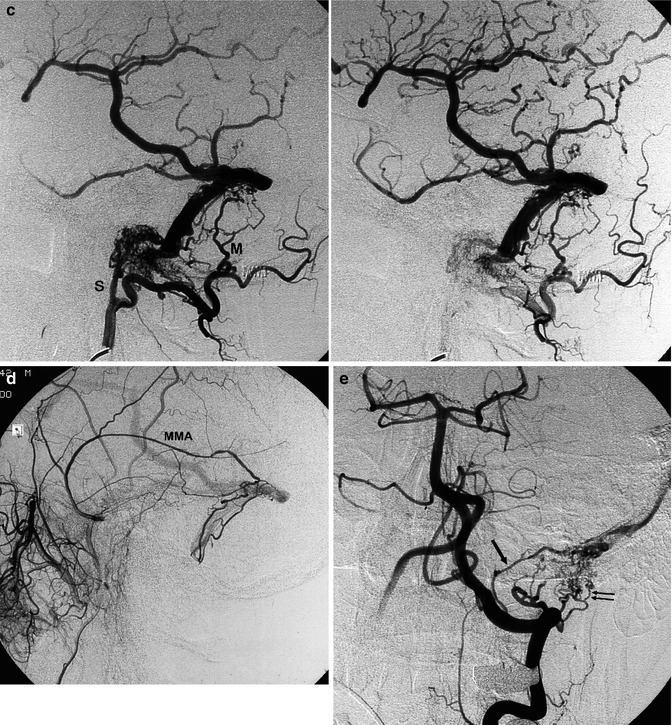
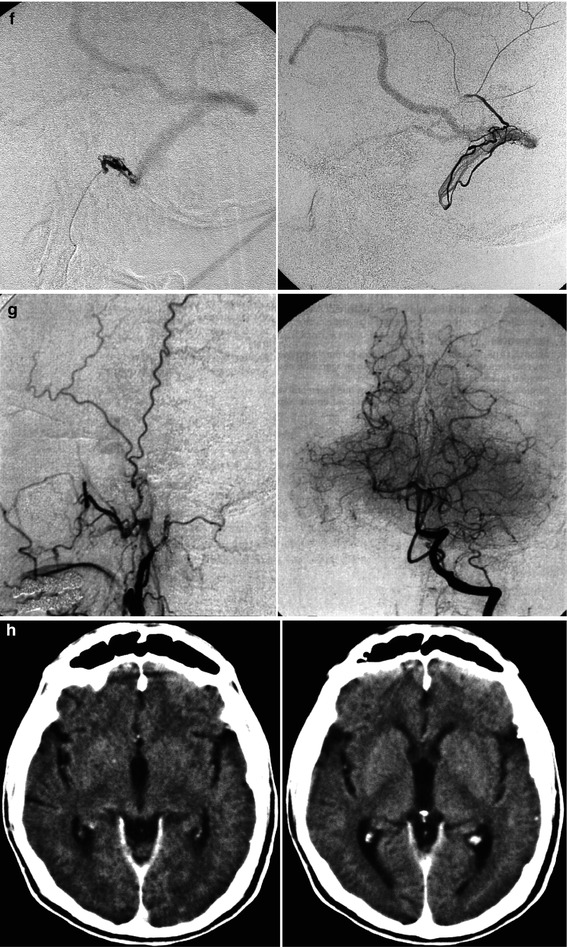
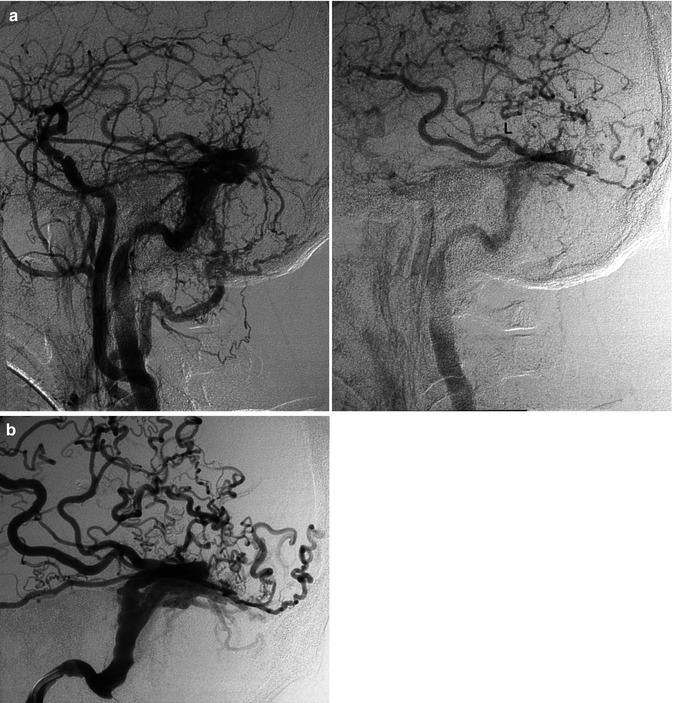
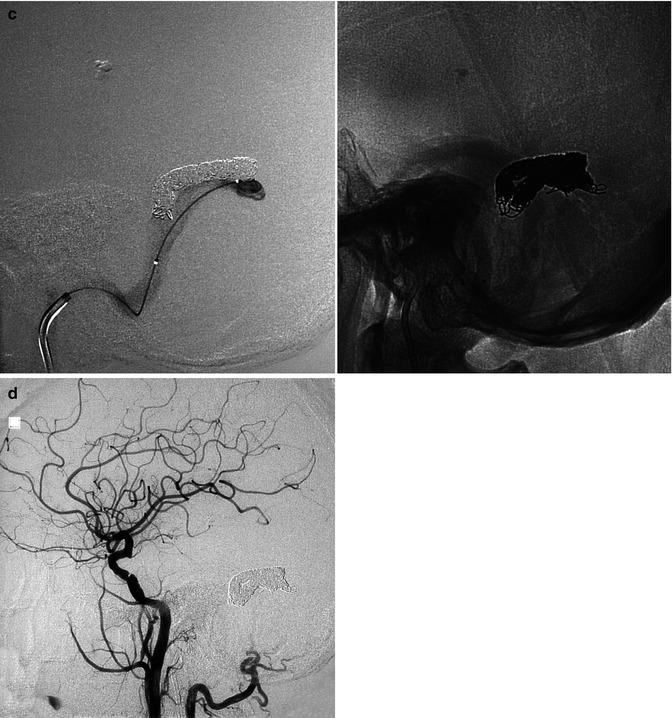
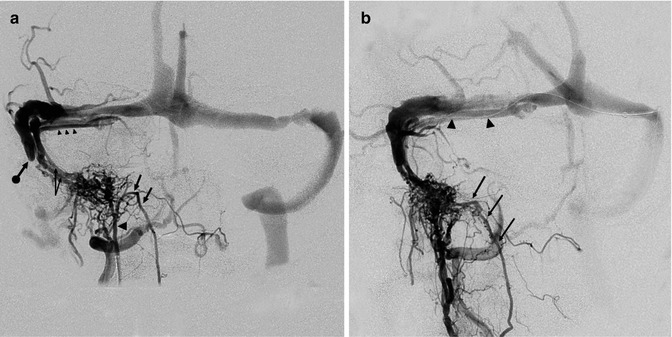



Fig. 13.1
Patient with mild aphasia and progressive cognitive disorder. CT (a) showed changes involving predominantly the white matter in the left temporal area, with rich irregular enhancement suggesting vascular malformation. Angiographic study showed the internal carotid artery (ICA) to be normal (b). On the angiogram of the external carotid artery (ECA), a dural arteriovenous fistula (DAVF) involving the left transverse sinus was demonstrated. The sinus was proximally and distally occluded, and a rich retrograde injection of the cortical temporal veins, including a large vein of Labbé, was present. The main supplying arteries (shown in c) were the occipital artery with its stylomastoid (S) and mastoid (M) branches, and the middle meningeal artery (MMA; shown in d). A partial supply came from the posterior meningeal artery (arrow) and C1 branches (double arrows) of the left vertebral artery (e). Selective distal catheterization of the MMA and stylomastoid artery (f) preceding the injection of acrylic glue followed by injection of polyvinyl alcohol (PVA) into the supplying branches of the vertebral artery, leading to complete occlusion of the fistula. ICA, ECA, and vertebral angiogram performed 3 months later (g) confirming occlusion of the DAVF. Normalization of the CT (h) corresponding to complete recovery of the patient


Fig. 13.2
Older patient with a known DAVF involving the transverse sinus (TS) treated with occlusion of several supplying branches of the ECA with PVA. The patient returned 6 months later suffering from the same symptoms, characterized by high bruit and headache. The carotid angiogram (a) showed complete recanalization of the fistula, involving the TS, which was proximally occluded. A rich retrograde injection of temporal veins, including a large vein of Labbé (L), was also present. On the venous phase of the ECA angiogram (b), retrograde injection of the cortical veins is evident. Note also the duplication of the sinus. The sigmoid sinus had already been catheterized. A microcatheter was advanced into the distal TS. The coils were placed first in the superior segment of the duplicated sinus (c1) and then in the inferior (c2). Control angiogram showing complete occlusion of the fistula (d)

Fig. 13.3
Fistula at the level of the right SiS. (a) Selective study of the APhA. AP view. The enlarged APhA (arrowhead) supplies the fistula. Through the hypoglossal branch (arrows), there is a large connection with the radiculomeningeal branch of the VA. The venous drainage occurs through two dural venous channels (arrows with angle) converging to a dural sinus (accessory sinus, arrowheads) running parallel to the main TS. There is a connection between the accessory sinus and the main TS. This latter seems distally to be completely occluded (arrow with dot). There is a retrograde injection of the left TS, the SS, and the SSS. (b) Oblique view: the anastomosis (arrows) between the hypoglossal branch of the APhA and the radiculomeningeal branch of the VA is better recognizable. A microcatheter has been advanced in the accessory dural sinus (arrowheads) and further in the two distal dural channels where coils were placed occluding the fistula
Considering especially the venous way of treatment, a particular aspect which has progressively become evident is the changes involving the sinuses which appear not rarely divided in two or more compartments (Piske et al. 2005). This finding is frequently recognized in fistulae involving TS and SiS. Why this occurs is not known. It has been suggested (Piske et al. 2005) that this could be related to a partial thrombosis of the sinus followed by its recanalization and formation of two or more separated venous channels. An accessory dural sinus separated but communicating with the main sinus can also develop and become the main venous drainage. These possible patterns should be taken into account since they are important in the endovascular treatment of the fistulae in this area (Figs. 13.2 and 13.3).
2.
DAVFs involving the cavernous sinus (CS) are the second-most frequent fistulas (Awad 1993; Cognard et al. 1995). Women are predominantly affected. The fistula can be uni- or bilateral. Also in cases of a unilateral shunt, a bilateral supply may be present. It is common for many feeders to arise from the distal internal maxillary artery (IMA), APhA, MMA, and cavernous portions of the ICA, uni- or bilaterally. More rarely, only branches of the ECA or ICA can be involved (Figs. 13.4 and 13.5). Considering the venous drainage, this can show different patterns. Indeed, as we have described in Sects. 2.3 and 9.3.10, the involved network of veins running in “the space of the cavernous sinus” can have and develop different connections depending on their location. Since the anterior part is connected with the superior ophthalmic vein (SOV) and inferior ophthalmic vein (IOV), a fistula in this sector will create a drainage to these vessels. It should be noted that the IOV connects with the pterygoid plexus. A fistula posteriorly located will be characterized by a drainage into the inferior petrosal sinus (IPS) and superior petrosal sinus (SPS) since this posterior location communicates with these venous channels (Cheng et al. 1999; Agid et al. 2004). Such a situation can occur when the two compartments do not communicate to each other or the links are minimal. A dominant drainage can also occur when one of the routes (ophthalmic veins or IPS, Fig. 13.4) is occluded by thrombosis, which leads to a rerouting of the drainage. In other cases, the two compartments probably are largely in communication, and so both draining patterns are present. Since the CSs are connected by a large intercavernous anastomotic channel, the contralateral sinus can also be involved.
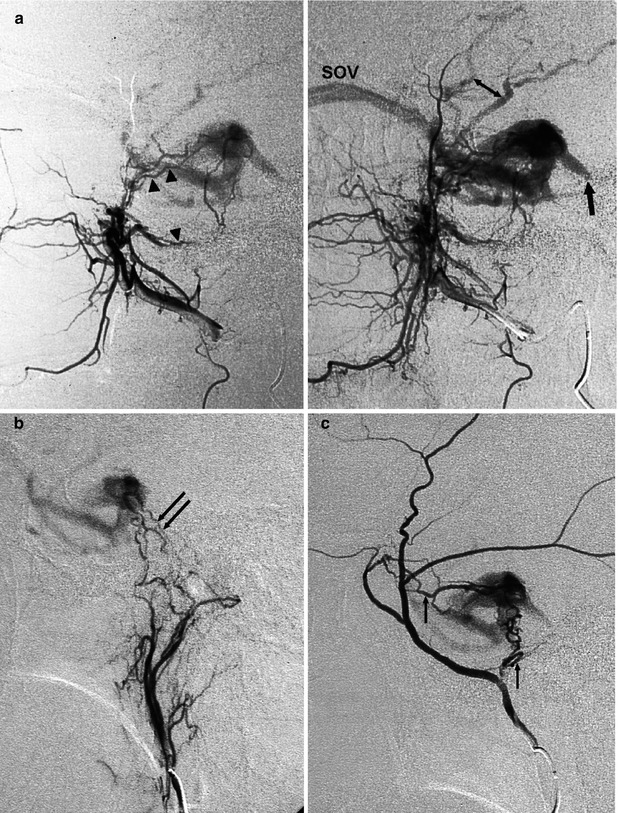
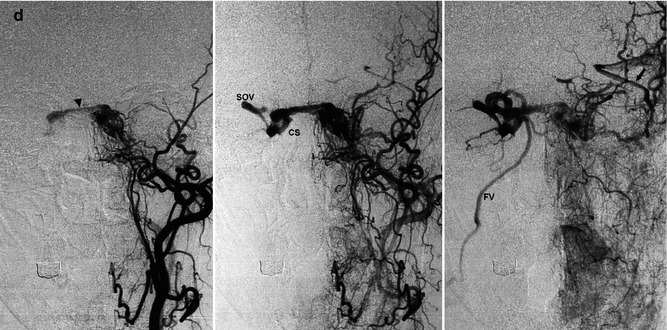
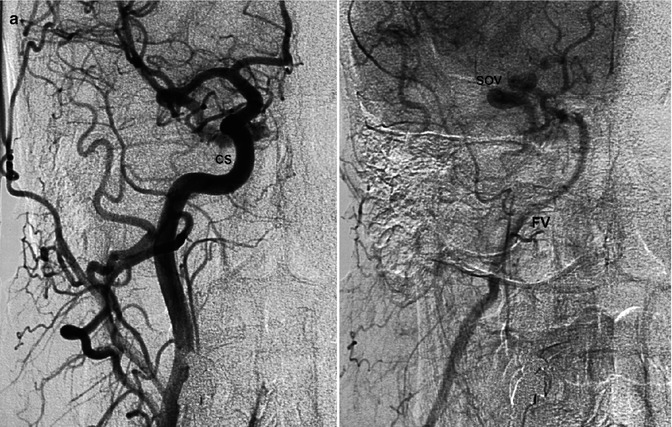
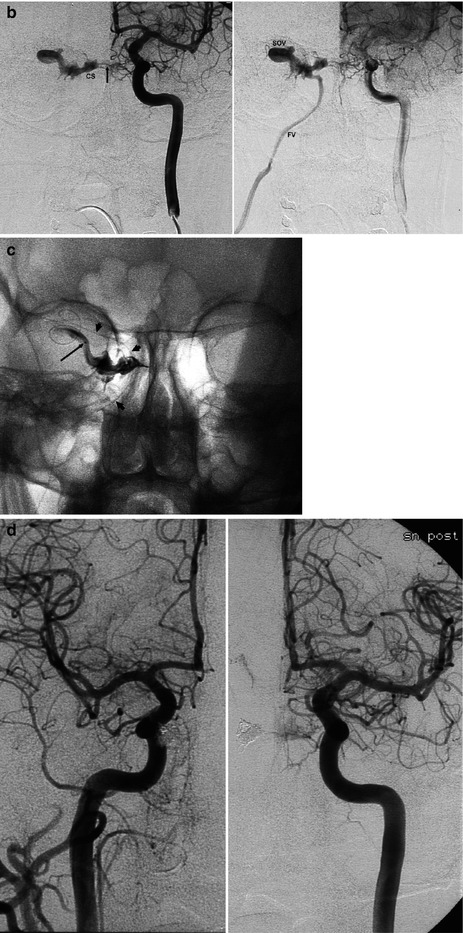


Fig. 13.4
Older woman presenting with bilateral exophthalmos, chemosis, and diplopia. The angiographic study of the bilateral ICA, ECA, and vertebral artery revealed DAVF at the level of the left cavernous sinus (CS) supplied by branches of the distal internal maxillary artery (IMA) and ascending pharyngeal artery. (a) Lateral angiogram of the distal left IMA showing the involvement of the foramen rotundum (arrowheads) and vidian arteries (arrowhead). Partial injection of the inferior petrosal sinus, which appears to be thrombosed (arrow). There is also a partial retrograde injection of the middle cerebral veins (bidirectional arrow). (b) Lateral angiogram of the ascending pharyngeal artery (APhA), showing the supply through its clival branches (arrows). (c) Lateral angiogram of the MMA involved with several branches (arrows). (d) ECA angiogram, AP view, showing involvement of the left cavernous sinus. Through intercavernous anastomoses (arrowhead), injection of the right cavernous sinus (CS) of the right superior ophthalmic vein (SOV) and further of the facial vein (FV). The left ophthalmic vein was not injected, probably thrombosed. Middle cerebral veins (arrow). Occlusion with PVA of the supplying arteries was performed, followed by treatment with low doses of heparin. Clinical improvement


Fig. 13.5
Middle-aged man with sudden onset of exophthalmos and diplopia due to a cavernous fistula located in the right sinus, supplied mainly by cavernous branches of both ICAs. There was a minimal involvement of the ECA. The drainage occurred anteriorly in the superior ophthalmic vein and further in the facial vein. This venous route was used to occlude the fistula with coils. (a) Right carotid angiogram, AP view. Cavernous sinus (CS), superior ophthalmic vein (SOV), facial vein (FV). (b) Left carotid angiogram, AP view. Left cavernous branches of the ICA supplying the fistula (arrow). Right cavernous sinus (CS), right superior ophthalmic vein (SOV), facial vein (FV). (c) Injection of contrast medium into the ophthalmic vein, near the cavernous sinus, during selective catheterization with microcatheter (arrows). The small arrow shows the distal microcatheter during its progress. (d) Right and left carotid angiogram post treatment
More infrequently, retrograde injection of the pial veins can occur through the several connections linking the plexus in the CS with the pial veins (Fig. 13.6). The possible involvement of the pial veins is as follows:
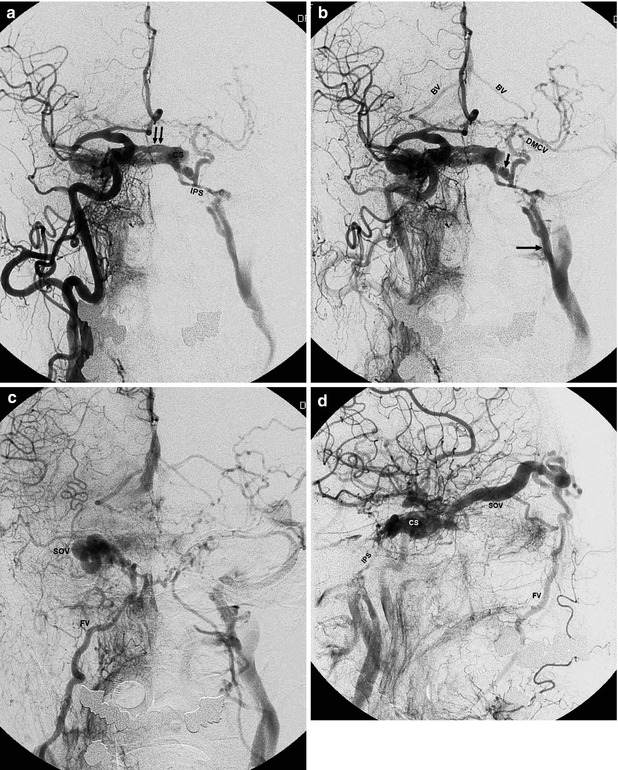


Fig. 13.6
Large DAVF involving the right cavernous sinus (CS) in an older woman with bilateral exophthalmos and diplopia. Typically, several branches of the IMA, APhA, and MMA and intracavernous branches of the ICA bilaterally are involved. The patient had already been treated with distal occlusion of branches of the ECA. Reappearance of symptoms after a short period of clinical improvement. Right carotid angiogram, AP view: (a), (b), and (c). There is immediate filling of the right CS. Through large intercavernous anastomoses (double arrow), there is rapid filling of the left CS, then of the inferior petrosal sinus (IPS) and jugular vein. The IPS does not enter the jugular bulb but the jugular vein more distally (arrow).There is a large anastomosis between the left CS and paracavernous sinus (short arrow) and retrograde filling of the deep middle cerebral vein (DMCV) and then of the basilar vein (BV) bilaterally. In the later phase (c), drainage through the ophthalmic vein and right dilated facial vein (FV) is evident. Right carotid angiogram, lateral view (d) showing drainage in the dilated superior ophthalmic vein (SOV), continuing to the facial vein (FV). Filling of the contralateral IPS. Selective catheterization (e) of the right cavernous sinus, advancing the microcatheter to the right jugular vein, then to the facial vein, and finally to the ophthalmic vein. Almost complete occlusion of the fistula obtained by placing coils in the right CS
The superficial and deep middle cerebral veins can drain into the venous plexus of the CS and paracavernous sinus (PCS), and so they can be retrogradely filled. It should be noted that the PCS is connected with the pterygoid plexus.
The basal vein (BV) can be retrogradely filled following involvement of the deep middle cerebral vein, which is normally a tributary of the BV. The CS is connected through a bridging vein with the pontomesencephalic vein. This latter communicates with the peduncular vein, which is a tributary of the BV. Furthermore, the injection of the SPS can lead to involvement of the petrosal vein, which is connected with the lateral mesencephalic vein, and then with the BV or posterior mesencephalic vein.
Many cerebellar veins, which can be retrogradely injected, also converge at the petrosal vein.
All these pathological venous drainages explain the clinical symptoms, which are proptosis, chemosis, nerve palsy, and pain (Vinuela et al. 1984; Cheng et al. 1999). Glaucoma and involvement of visual acuity can also occur probably due to impairment of the venous drainage of the central retinal vein. All these clinical symptoms can be present in all the DAVFs, involving primarily or secondarily the CS (see also parts 5 and 6 of the Sect. 13.7 and Fig. 13.20). In some cases, the symptoms become more serious because of hemorrhage or venous congestion in the temporal area, basal ganglia, brainstem, and cerebellum (Takahashi et al. 2001; Suh et al. 2005; Kim et al. 2006; Kiyosue et al. 2008; Miyamoto et al. 2009). The treatment of these fistulas is commonly endovascular. The arterial or venous route can be chosen depending on the morphology and clinical condition (Figs. 13.4, 13.5, and 13.6) (Halbach et al. 1989; Quinones et al. 1997; Cheng et al. 1999; Benndorf et al. 1999, 2001a; Agid et al. 2004; Kirsch et al. 2006; Kato et al. 2007; Yung et al. 2011; Yu et al. 2011).
A type of fistula, which is very rare, is that developing in the middle cranial fossa with involvement of the venous channels present in this area, but not the CS. The venous pattern can take different aspects, depending on the variants of the drainage of the sphenoparietal sinus (SpS), SMCV, and SOV, as described in Sects. 9.1.1.2, 9.3.9, and 9.3.11. An example is presented in Fig. 13.7a, b. The fistula involved the paracavernous sinus and further the SpS and the SOV, mimicking clinically a CS fistula. A few cases of this kind of fistula, frequently traumatic, are reported in the literature (Pakarinnen 1965; Theron et al. 1975; Bradac et al. 1981b; Freckmann et al. 1981; Unterhofer et al. 2009; Shi et al. 2013). Another example is shown in Fig. 13.7c–e. This was spontaneous fistula involving the SpS in its course on the floor of the middle cranial fossa continuing in its tentorial drainage which was thrombosed. There was a retrograde injection into a dilated SMCV.
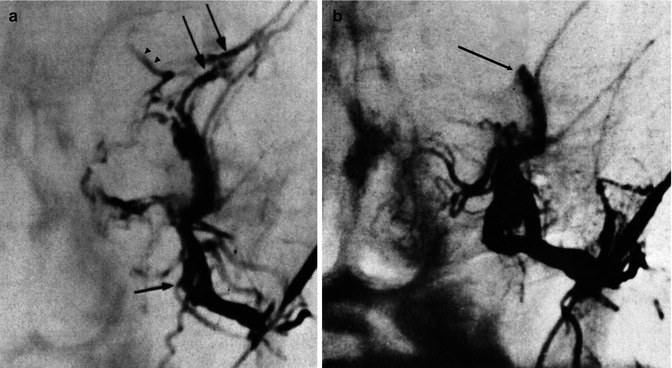
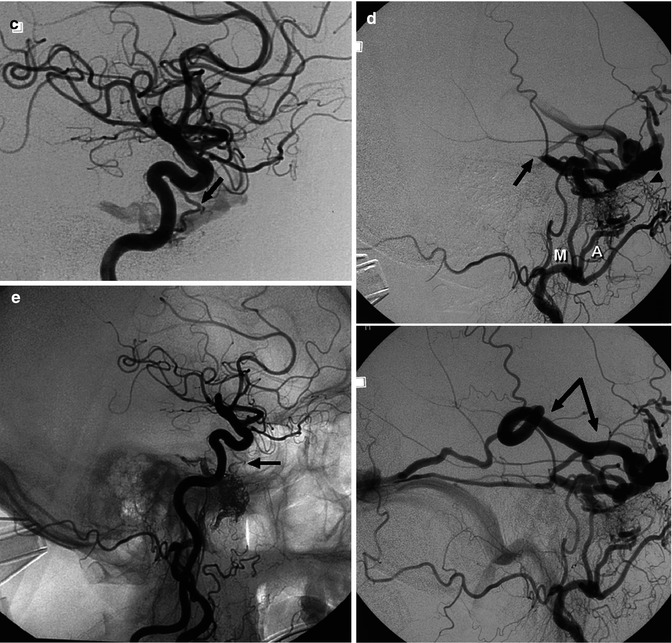


Fig. 13.7
(a, b) Fistula involving the sphenoparietal sinus. A few days after a blunt head trauma, a clinical syndrome typical of a CS fistula developed. ICA angiogram was normal. (a) Selective angiogram of the MMA (arrow) showed a network of veins at the skull base continuing in the sphenoparietal sinus (arrows) and in the SOV (arrowheads). The CS is not involved. (b) Control angiogram post endovascular treatment using PVA. (c–e) Another patient with fistula involving the middle cranial fossa presenting with a temporal hematoma. (c) ICA lateral angiogram showing the involved dilated ILT (arrow). (d) ECA lateral angiogram, early and later phases. The fistula is supplied by the middle meningeal artery (M), accessory meningeal artery (A), and forum rotundum artery (arrowhead). The venous connection seems to involve predominantly the SpS in its course on the floor of the middle cranial fossa. It is thinkable that, in this case, the SpS drained in the TS through tentorial channels. Two are recognizable. The largest is occluded (arrow). The drainage occurred through a retrograde injected SMCV (arrow with angle). Treatment with Onyx injected in the MMM was performed. There was a risk of a retrograde injection of the embolic material in the ILT and in the ICA. A continuous control due to ICA injection was performed during the treatment. (e) Common carotid angiogram post treatment showing the occlusion of the fistula. Small ILT (arrow)
3.
Get Clinical Tree app for offline access

DAVFs of the SSS are less frequent. They can be of type 1, but it is not seldom for them to be of types 2 and 3 (Figs. 13.8 and 13.9). In type 2, the venous reflux can be extensive and involve a large part of the cortical veins of the hemisphere. In type 3, the drainage in the sinus is mediated by dilated cortical veins, which enter the sinus after a fairly long, tortuous course. Branches of the ECA, mainly the MMA, are commonly involved, frequently bilaterally. Transosseous branches of the superficial temporal artery and occipital artery can be present. The anterior meningeal artery, arising from the ophthalmic artery, and the meningeal branches of the VA may sometimes supply the shunt. Epileptic seizures, hemorrhage, and/or signs of intracranial hypertension, such as headache and cognitive disorders, can be present (Halbach et al. 1988; Riva et al. 1991). Surgical disconnection of the shunt or selective embolization with acrylic glue (Collice et al. 2000; Van Dijk et al. 2004; Rodesch et al. 2009) may be effective treatments.
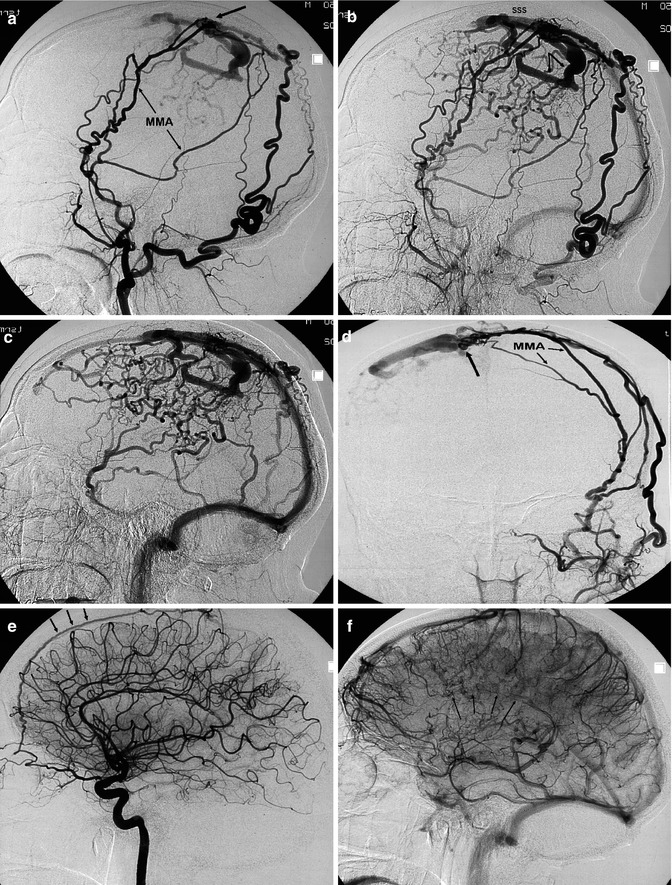
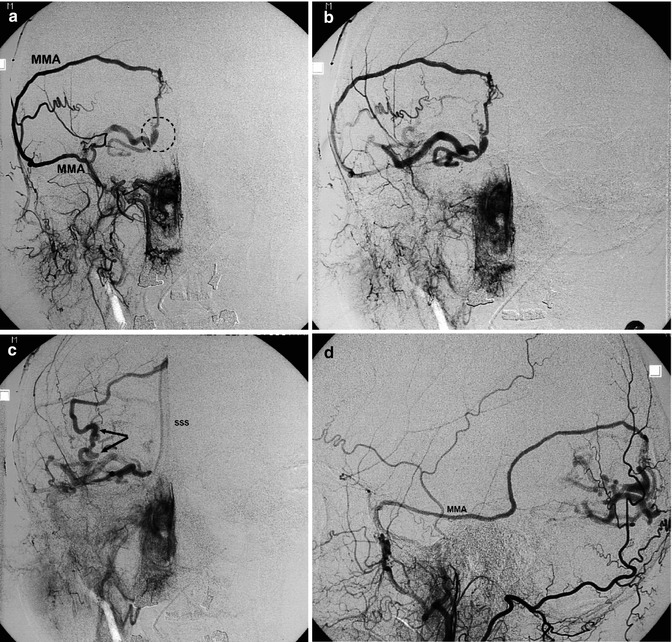
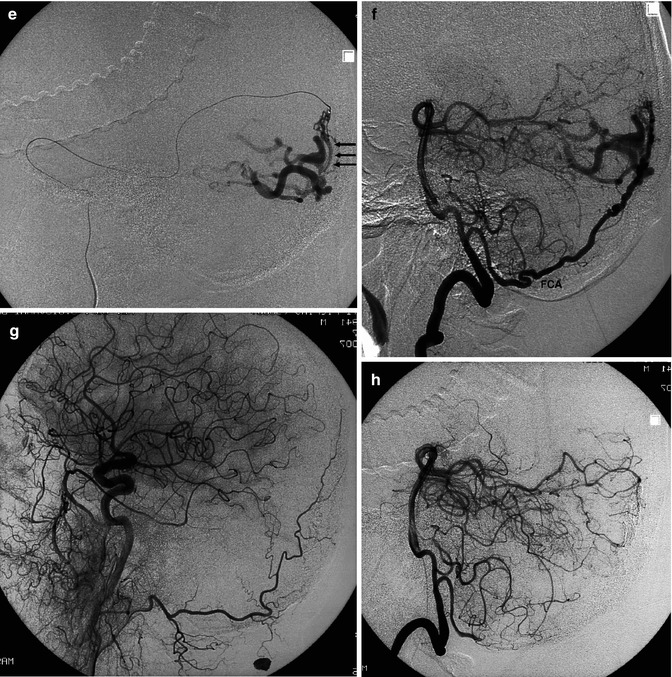

Fig. 13.8
Middle-aged patient with increased headache and suspected epileptic seizures. The angiographic study shows DAVF located in the right parietal area, close to the SSS. Right (a–c) and left (d) ECA angiograms showing the DAVF supplied by branches of both middle meningeal arteries (MMA) and by transosseous branches of the occipital arteries. Site of the shunt (arrow). The drainage occurs in the enlarged cortical veins (inclined arrows), which, after a tortuous course, enter the SSS. There is retrograde injection of a large part of the cortical veins of the right hemisphere. On the right ICA angiogram (e), a minimal supply through the anterior meningeal artery (arrows) is visible. In the venous phase (f), congestion in the venous drainage explains the irregular and poor injection of the veins (arrows). The patient underwent surgical treatment


Fig. 13.9




Older patient suffering from acute headache. CT showed a suspected vascular malformation in the right occipital area. Angiography indicated a DAVF located in the right occipital area, close to the SSS. Angiogram of the right ECA, AP view (a–c) showing the middle meningeal artery (MMA) supplying the malformation. Site of the shunt (circle). The venous drainage occurs through the cortical veins (inclined arrow), which, after a tortuous course, enter the superior sagittal sinus (SSS). Lateral angiogram of the ECA (d) followed by selective catheterization of the MMA (e), showing the site of the shunt (arrows) and venous drainage. There was no involvement of the ICA, but a supply through the falx cerebelli artery (FCA), arising from the intracranial vertebral artery, was visible on the vertebral angiogram (f). Endovascular treatment with Onyx injected into the MMA allowed complete occlusion of the fistula. Carotid and vertebral control angiograms (g) and (h) post treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


