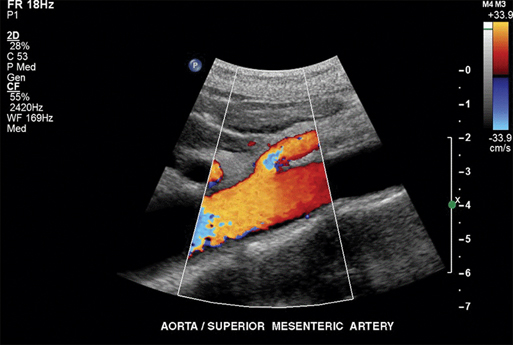
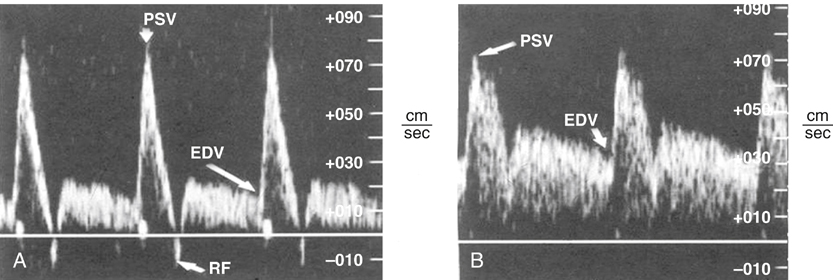
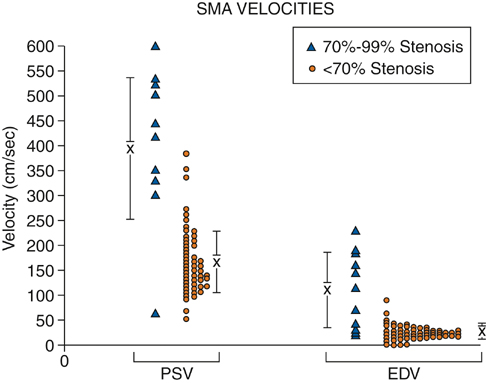
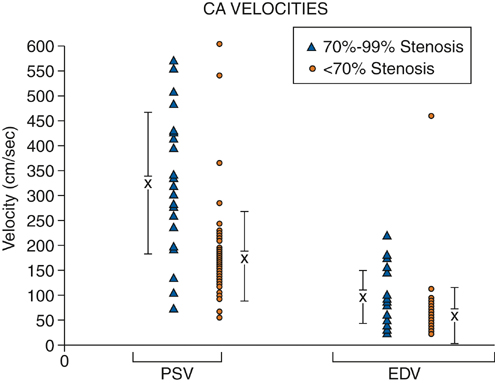
To minimize bowel gas interference, mesenteric duplex scanning is best performed early in the day with the patient fasting. The patient is supine, and the head of the bed is elevated to 30 degrees. B-mode imaging and Doppler insonation of the visceral vessels is required. Doppler frequencies are typically in the range of 2.0 to 5.0 MHz. Gray-scale and color-flow imaging (Figure 1) are used to identify and follow the selected vessel segments and to note the presence or absence of any disease process within the vessel lumen. Doppler evaluation quantifies the severity of the disease and should include assessment for the presence or absence of flow and, when flow is present, evaluation of peak systolic velocity (PSV) and end diastolic velocity (EDV). Spectral analysis should be obtained in all vessel segments. Measurements should be obtained proximal, throughout, and distal to any flow disturbance identified. All spectral derived velocity information should be derived with a Doppler insonation angle between 45 and 70 degrees. Angles of insonation exceeding 70 degrees result in significant artificial elevation of velocities. Fasting SMA and CA waveforms differ. Fasting peak-systolic velocities in the SMA tend to be higher than those in the celiac artery. Fasting end-diastolic velocities also tend to be lower in the SMA than in the CA. Peak systolic velocities in the SMA in angiographically normal vessels vary from 125 to 170 cm/sec. A reverse flow component is often present at the end of systole in the SMA but not in the CA (Figure 2). The increased diastolic flow present in the CA compared to the SMA likely reflects the decreased end organ resistance of the hepatic and splenic circulations versus that of the fasting intestinal circulation. A 70% to 100% CA stenosis was found in 24 patients, and 76 patients had less than 70% CA stenosis. There were 14 patients with 70% to 100% lesions in the SMA, and 85 SMAs had less than 70% angiographic stenosis. Comparing PSVs and EDVs in the patients with less than 70% angiographic stenosis versus those with more than 70% angiographic stenosis, PSVs and EDVs were found to be higher in the patients with greater than 70% stenosis in the CA and/or SMA (Figures 3 and 4).
Duplex Scanning in the Diagnosis of Splanchnic Artery Occlusive Disease
Technique of Mesenteric Duplex Scanning
Fasting Doppler Mesenteric Artery Waveforms
Fasting Duplex Criteria for Mesenteric Artery Stenosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree