14 Drug-Coated Balloons
 Introduction
Introduction
Coronary angioplasty was introduced into clinical use by Andreas Grüntzig in 1977.1 In the field of coronary intervention the most important advance has been the introduction of stents. Stenting overcomes acute recoil and dissection as well as longer-term negative remodeling but not restenosis due to continued neointimal proliferation. Local intravascular drug delivery by drug-eluting stents appears to have addressed this cellular basis of restenosis. However, stents cannot be implanted at all coronary sites where neointimal proliferation may limit the long-term benefit of angioplasty, such as small vessels and bifurcations, and drug eluting stents have not been found to be effective in the treatment of peripheral vascular disease of the femoropopliteal territory. In the coronary arteries, sometimes delayed or incomplete reendothelialization with the need for long-term dual antiplatelet therapy to reduce the risk of late stent thrombosis can limit the use of this technology in certain patients. Sustained drug release seems to be essential for stent-based local drug delivery owing to the inhomogeneous drug distribution from a drug-eluting stent to the arterial wall,2 the time course of the inflammation related to the initial trauma of the procedure, as well as the provocation of neointimal hyperplasia due to the implanted prosthesis. About 75% to 85% of the stented vessel wall area is not covered by the stent struts, resulting in low tissue levels of the antiproliferative agent in these areas. Cell culture experiments indicate that low drug concentrations require much longer exposure times to achieve sufficient inhibition of cell proliferation than do higher concentrations.3 Therefore high drug concentrations on the stent struts, including a controlled and sustained release, are mandatory for stent-based local drug delivery,4 with the consequence of delayed and incomplete reendothelialization of the stent struts. Autopsy studies show that even beyond 40 months, drug-eluting stents are not always fully covered by endothelium.5 Furthermore, the polymeric matrices on the stent that are meant to control the release kinetics of the antiproliferative drug can induce inflammation and thrombosis.6,7 On the other hand, incomplete suppression of neointimal hyperplasia at the stent margins or between the struts may limit the efficacy of drug-eluting stents.2,8 Alternative approaches to overcome the limitations of drug-eluting stents have included avoiding a sustained drug release from stent struts to allow for earlier reendothelialization, bioerodable polymers or nonpolymeric release mechanisms (such as surface-modified stent struts), and thinner struts requiring less coverage. Antiproliferative taxanes such as paclitaxel seem to be suitable for the prevention of local intravascular restenosis because of their high lipophilicity and tight binding to various cell constituents, resulting in effective local retention at the site of delivery.3 The addition of a contrast agent surprisingly resulted in a solubility of taxanes far beyond the concentrations applied in previous investigations.9 In the porcine coronary model, the intracoronary bolus administration of a taxane-contrast medium formulation led to a significant reduction of neointimal formation after experimental coronary stent implantation despite the short application time.10,11 Paclitaxel in a contrast agent was better tolerated and led to higher local tissue concentrations than diluted Taxol, indicating the impact of additional compounds for local drug transfer.12 The surprising discovery was that sustained drug release is not a precondition for long-lasting restenosis inhibition. In 2001, the basic premise of a more lesion- than vessel-specific method of intramural drug delivery became embodied in the concept of a drug-coated balloon. By coating paclitaxel onto the surface of a conventional angioplasty balloon used to dilate the stenotic artery, an exclusively local effect could theoretically be achieved, with the drug transferred to the dilated segment as the balloon was inflated. In this way, an effective local drug concentration is achieved with very low systemic exposure. However, several properties of the balloon coating are crucial for ensuring effective drug delivery to the target site, including (1) its form on the balloon surface; (2) the homogeneity of distribution along the surface of the balloon; (3) its stability during production, handling, and storage; (4) the degree of premature loss while transiting to the target vessel segment; (5) the ability to release during balloon expansion; (6) the transfer efficiency to the vessel wall; and (7) the amount of particulate material released to the distal circulation.
 Preclinical Data
Preclinical Data
Speck and colleagues, using various coating procedures, coated conventional coronary balloon catheters with different doses of paclitaxel. The paclitaxel dose on the coated balloons was 1.3 to 3 µg/mm2, corresponding to a total dose of approximately 220 to 650 µg paclitaxel, depending on the balloon size. About 10% of the initial amount of paclitaxel on the balloon was lost while the catheter was being advanced to the lesion through the hemostatic valve and the guiding catheter and about 80% of the dose was released during inflation. Most of the dose released at the target site is distributed as particulate distally in the bloodstream, with less than 20% being directly taken up into the vessel wall. Thus paclitaxel-coated balloons deliver a dose to the target site in a very short time, and this dose is higher than that released by stents over the course of weeks. At 5-week follow-up, the implantation of stents premounted on paclitaxel-coated balloons was found to have caused a marked dose-dependent and statistically significant reduction in late lumen loss and an equally impressive statistically significant increase in minimal lumen diameter compared with controls. Quantitative coronary angiography revealed no edge effects or signs of malapposition or aneurysm. Histomorphometry showed a statistically significant increase in lumen diameter and lumen area and a corresponding decrease in maximal neointimal thickness and neointimal area in the vessels treated with paclitaxel-coated balloons (reduction of neointimal area by 63% in the paclitaxel-coated balloon group vs. the uncoated balloon group).12,13 Furthermore, the drug is more evenly distributed on the vessel surface compared with that delivered by a drug-eluting stent.14 However, studies suggest that the amount of paclitaxel in the arterial tissue varies widely depending on the dose of drug on the balloon and particularly on the coating formulation. An adequate inhibition of neointimal proliferation was observed only when balloons were coated with paclitaxel mixed with the contrast agent iopromide dissolved in acetone. The effect was markedly lower when ethyl acetate was used as a solvent without iopromide. The difference in efficacy of these two coating formulations may be primarily explained by the presence of the hydrophilic iodinated contrast medium in the case of the acetone version, thus suggesting that a proper solubilizing agent is important.15
Paclitaxel admixed with a small amount of the hydrophilic contrast medium iopromide (Ultravist) has also been denoted as Paccocath. These balloons are standard angioplasty balloons coated with a paclitaxel dose of 3 µg/mm2 of balloon surface. The situation in the peripheral arteries is not directly comparable with that in the coronary arteries, and treatment is much more complex in several respects. Compared with the coronary arteries, the incidence of restenosis in the superficial femoral artery is even higher and can reach up to 50% within the first 6 months after intervention. Given the clinical need, it was very encouraging when Albrecht et al. developed early preclinical data demonstrating that local intra-arterial administration of paclitaxel using drug-coated balloons or an admixture of paclitaxel to contrast medium could inhibit in-stent stenosis of peripheral arteries in the porcine overstretch model: in-stent stenosis in the control group was 38% ± 20% (uncoated balloons). In the treatment groups, it was reduced as follows: treatment group I (balloons coated with 330 µg paclitaxel), 18% ± 22%; treatment group II (balloons coated with 480 µg paclitaxel), 12% ± 18%; and treatment group III (6.4 mg paclitaxel dissolved in 50 mL iopromide 370 + 5 mL ethanol), 18% ± 20% (P < 0.05).16 Cremers et al. subsequently evaluated the effects of various inflation times (10, 60, and 2 × 60 seconds) on the efficacy of restenosis inhibition and the safety of different doses (5 µg; 2 × 5 µg paclitaxel/mm2 balloon surface) in pigs. Treatment with a drug-coated balloon (5 µg paclitaxel/mm2 balloon surface with iopromide) for 10 seconds reduced the neointimal area to the same extent as contact with the vessel wall for two times 60 seconds (by 57% and 56%, respectively, compared with control). Furthermore, neointimal proliferation and all other parameters characterizing in-stent restenosis were not further decreased by inflating two drug-coated balloons (each containing 5 µg paclitaxel/mm2 balloon surface) in the same vessel segment for 60 seconds each. These results suggest that balloons coated with the paclitaxel iopromide formulation release most of the drug rapidly during the first seconds of inflation. Thus, the initial contact of the coated balloon membrane with the vessel wall appears to produce the desired effect of inhibiting neointimal proliferation. The results of this study indicate that it may be sufficient to inflate the balloon for a few seconds only to achieve adequate protection from restenosis. The results also show that doses of up to 10 µg paclitaxel/mm2 balloon surface applied by the inflation of two drug-coated balloons do not increase the risk of thrombosis or cause aneurysm.17
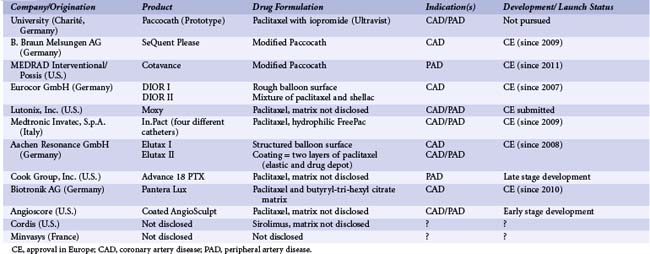
Since this initial research was published, several manufacturers have started commercializing or developing drug-coated balloons. Currently, paclitaxel is the drug of choice, the typical dosage being 3 µg/mm2 balloon surface. The critical factor enabling successful drug transfer is the formulation used to coat the balloon. Current products range from those with no additive and very tight binding of the drug to the balloon membrane to those applied in conjunction with contrast agents or other beneficial additives. A number of these developers have undertaken extensive research into this issue and believe that the formulation will be critical to successful product performance and adoption (Table 14-1). Still, only scarce data are available on drug-coated balloons other than the Paccocath. The matrix coating of the SeQuent Please balloon catheter (B. Braun Melsungen AG, Germany) for PTCA consists of a mixture of paclitaxel and iopromide, identical in composition to Paccocath. The preclinical data compare very well with the results from the Paccocath program. Granada and colleagues reported histological results showing the Cotavance coating (MEDRAD/Possis, USA, Bayer) an iterative coating formula based on Paccocath, to be superior to an uncoated balloon in treating coronary artery and superficial femoral artery lesions in pigs.17a In an additional pilot study, single or overlapping Cotavance balloons were compared with single nonoverlapping balloons coated with a contrast medium (Iopromide) without paclitaxel in a healthy porcine iliofemoral stent model. Balloon angioplasty was followed by self-expandable bare metal stent implantation. After 28 days, Cotavance balloons decreased neointimal proliferation in a dose-dependent manner when assessed by quantitative angiography (late lumen loss with Cotavance single 1.5 ± 0.7 mm vs. Cotavance overlap 0.7 ± 0.6 mm compared with contrast-coated control 1.7 ± 0.4 mm).18
FreePac (Medtronic Invatec, Italy) is a proprietary hydrophilic coating formulation with urea serving as the matrix substance. Urea is a nontoxic, ubiquitous endogenous compound commonly used in pharmacy; it is meant to enhance the release of paclitaxel during the short time of contact with the vessel wall. In the porcine coronary model, similar amounts of paclitaxel were transferred to the vessel wall with the Paccocath coating (214 ± 106 µg paclitaxel) and the FreePac coating (175 ± 101µg paclitaxel) 15 to 25 minutes after stent implantation. Twenty-eight days after balloon dilatation, the original Paccocath coating caused the known strong inhibition of neointimal formation in the porcine coronary model (minimal lumen diameter [MLD]: 2.7 ± 0.3 mm; late lumen loss: 0.3 ± 0.2 mm). The FreePac coating was equally efficacious and equally well tolerated (MLD: 2.7 ± 0.2 mm; late lumen loss: 0.4 ± 0.2 mm). The aim of another study was to determine the minimum effective dose and local toxicity at extremely high doses of the FreePac formulation. The balloons were coated with 1 to 9 µg paclitaxel/mm2 balloon surface. In the highest-dose group three balloons each coated with 9 µg paclitaxel/mm2 balloon surface were expanded in the same vessel segment. FreePac paclitaxel-coated balloon catheters efficaciously inhibited neointimal proliferation starting with the lowest dose tested (1 µg/mm2) and were well tolerated up to three times the preferred dose of 3 µg/mm2. Stent occlusions observed at the highest dose level and repeated treatment (3 × 9 µg/mm2) indicate that the limit of tolerance was reached.19
As early as 2007, a paclitaxel coated balloon catheter called DIOR received approval in Europe (CE mark). A study of first-generation DIOR balloon catheters (Eurocor GmbH, Germany) reported a tissue paclitaxel concentration of the dilated segment in porcine arteries 1.5 hours after dilation of 1.82 ± 1.60 µmol/L, which decreased significantly to 0.73 ± 0.27 (P = 0.03), 0.62 ± 0.34, and 0.44 ± 0.31 µmol/L at 12, 24, and 48 hours.20 In a direct comparison with the Paccocath balloon, the roughened DIOR balloon failed to produce statistically significant effects on angiographic measures of stenosis or morphometric parameters such as maximal neointimal thickness and luminal area. Use of the matrix-coated Paccocath balloon led to a highly significant (P < 0.01) reduction in all parameters, indicating neointimal proliferation compared with both uncoated control and DIOR at 28-day follow-up.21 Only about 50% of the drug coating was released from the roughened balloons during the recommended balloon inflation time of 45 to 60 seconds. In contrast, the iopromide matrix was found to release the full amount of the drug (4.5% ± 0.7 % of the total paclitaxel dose on balloons after the procedure), which may contribute to its superiority in inhibiting restenosis. The second-generation DIOR II balloon is a coronary dilation balloon for human use with a paclitaxel coating of 3.0 µg/mm2 on the balloon surface; this is applied using a completely different coating technique. The drug is mixed with shellac, which is composed of a network of hydroxy fatty acid esters and sesquiterpene acid esters with a molecular weight of about 1,000. The 1 : 1 mixture of paclitaxel and shellac is coated onto regular balloon catheters. A balloon inflation time dependency study in the porcine model of coronary artery overstretch showed almost maximal tissue paclitaxel concentrations after balloon inflation times of 30 seconds and release of 75% of the drug from the balloon surface, which resulted in an up to 20-fold higher tissue concentration compared with the first-generation DIOR. Two weeks after overstretch injury, histomorphometry showed significantly smaller neointimal hyperplasia and neointimal thickness in the DIOR group compared with the conventional uncoated balloon group. Consequently the area of the coronary artery lumen was larger in the DIOR-treated arteries compared with those treated with the conventional balloon (1.20 ± 0.27 mm2 vs. 0.5 ± 0.22 mm2, P
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree